Abstract
During the past two decades peripheral blood stem cells have become the favored graft source for HSCT with 80 % of allogeneic and almost 100 % of autologous HSCT performed with mobilized blood. The critical role of the interaction between the chemokine receptor CXCR4 and its chief ligand CXCL12 for retention and migration of hematopoietic stem and progenitor cells (HSPC) has been well established. Interference with CXCR4/CXCL12 signalling iscurrentlybeing exploited as a strategy to mobilize HSPC indirectly with the most clinically relevant mobilizing agent to date, G-CSF as well as directly with the bicyclam CXCR4 antagonist Plerixafor (AMD3100).In this study, qualitative and quantitative effects of long-term pharmacologic inhibition of CXCR4/CXCL12 axis within the HSPC compartment were investigated in healthy C57BL/6 mice using the non-peptidic small molecule CXCR4 antagonists Plerixafor and ALT1188 along with the Protein-EpitopeMimeticsInhibitor POL5551.
Up to 12-14 fold higher mobilization efficiency was achieved by applying the antagonists via two weeks of continuous infusion (up to 8-10x104 CFU-C and LSK/ml) as compared to bolus treatment (4-6x103 CFU-C and LSK/ml) or 5-day course of G-CSF (3-6x103 CFU-C/ml).Despite dramatic increase in numbers of circulating HSPC, the BM HSPC pool dis not decrease; in fact it expanded up to 2-4-fold compared to steady state reservoir (sham-operated control mice) as measured by immunophenotypical (LSK SLAM) and functional (e.g. serial competitive transplantation) properties of the cells. Thus, in contrast to genetically CXCR4 ablatedHSPC, the reversible long-term blockade of the receptor did not diminish the long-term repopulating capacity of HSPC.
Cell cycle analysis showed a 2-3-fold increase in cycling activity of BM HSPC: only 10-20% of LSK and 30-40 % of LSK SLAM cells were found to be quiescent (in G0 phase of the cell cycle) after two weeks of CXCR4 antagonist infusion versus 50-60 % of LSK and 70 % of LSK SLAM found in G0 under homeostatic conditions. This increased proliferation was very similar to the one induced transiently at day 3 G-CSF treatmentand would conceivably explain the sustained mobilization without concomitant depletion of the BM HSPC pool. Profiling of differentially treated BM HSC (LSK SLAM) via microarray analysis did not reveal substantial effects of CXCR4 inhibitor infusion on the expression signature.
Ofnote, major cytological changes typically associated with G-CSF induced mobilization, e.g. depletion of bone lining osteoblast lineage cells and macrophages, were not detected in continuous infusion of POL5551 exposed BM suggesting limitedeffects within the BM niche compartment. Moreover analysis of the BM HSPC after different washout periods at the end of continuous infusion treatment revealed a rapid (within 1-3 days after discontinuation of infusion) reestablishment of steady state HSPC numbers in the BM.Our data suggest that prolonged pharmacologic blockade of the CXCR4/CXCL12 axis using multiple small molecule inhibitorsrepresents an approach thatreleasesHSPCwith efficiency superiorto any other knownmobilization strategybut also may serve as an effective method induce cell cycling and thus expand BM HSPCs.
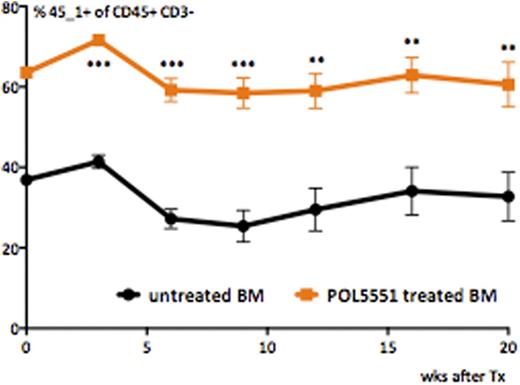
Competitive transplantation of POL5551 treated andcontrol BM (n=5 recipients per group, mean±SEM)
Competitive transplantation of POL5551 treated andcontrol BM (n=5 recipients per group, mean±SEM)
Levesque:GlycoMimetics: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal