Abstract
Introduction
β2-glycoproteinI (β2GPI) is the primary antigen for antiphospholipid antibodies (Ab), and Ab to β2GPI are associated with thrombosis and recurrent fetal loss.β2GPIis comprised of 5 "sushi" domains. Complex disulfide bonding renders β2GPI a challenging protein to producerecombinantlyin high yield and most studies have utilized domain-deletion mutants produced on a lab scale for structure-function analyses.β2GPIalso has a complex tertiary structure, and is reported to circulate in a "circular" form that may "open" to expose the antigenic site for β2GPIAbunder specific conditions. We developed a novel method to produce recombinant β2GPI in which replacement of the leader peptide allows large scale expression using a lentiviral system with one-step purification on heparin-sepharose. The ability of this protein to bind anti-β2GPI Ab was compared with that of plasma-derived (wild type, WT) β2GPI.
Methods
β2GPIcDNAwas cloned into pLentiCMV DEST. The β2GPI containing vector was used to transduce HEK293 cells with selection using puromycin. β2GPIwas purified from conditioned medium using HiTrapHeparin HP. Plasma β2GPI was purified using a protocol employing perchloric acid precipitation followed by heparin-sepharose and Mono-S chromatography.
To measure anti-β2GPI Ab, we analyzed plasma from 32 patients referred to the Cleveland Clinic Special Coagulation Laboratory for anti-β2GPI testing using the InovaQuanta-Lite ELISA. Normal plasma samples (n=15) were also analyzed at 1:100 dilution to determine cutoffs for anti-β2GPI positivity. Briefly, 96-well plates were coated overnight at 4° C with 2 µg/ml WT or recombinant β2GPI. After blocking β2GPI-coated plates with BSA, 50 µl of patient plasma at 1:10 and 1:100 dilutions were added to individual wells in quadruplicate. A standard curve for IgG binding to each plate was created using affinity-purified rabbit anti-β2GPI IgG at concentrations of 15, 31.25, 62.5, 125, and 250 ng/ml. After incubation for 30 minutes at room temperature, plates were washed three times and 100 µl of a 1:5000 dilution of goat anti-human IgG was added. After 30 minutes, wells were again washed prior to adding 100 µl/well of o-phenylenediamine dihydrochloride. Plates were read at 490 nm after 15 minutes following addition of 25 µl/well H2SO4. Results from different plates were standardized by extrapolating the amount of boundAb from the standard curve prepared on each plate.
To compare performance of recombinant β2GPI against WT β2GPI in ELISA we first evaluated correlation using recombinant and WT β2GPI by Spearman's test. The two sets of ELISAs were also compared using the Wilcoxon matched pairs test. ELISA readings were considered positive if they were >90th percentile on a curve established using 15 normal plasmas. Sensitivity and specificity of the assays was determined with respect to the results of the clinical assay.
Results
Recombinant β2GPI was produced in high yield (10-20 mg/L) and purified to homogeneity with a single heparin chromatography step; the purified protein migrated as a single band of ~50 kDon SDS-PAGE with a characteristic increase in Mr upon reduction.
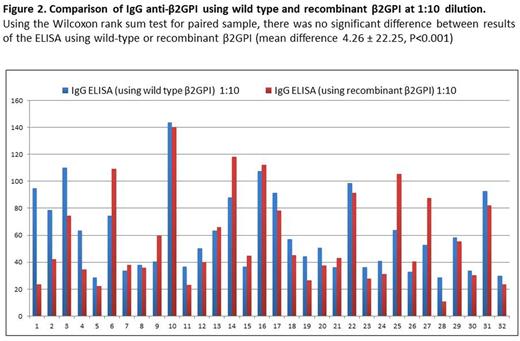
Anti-β2GPI IgG ELISA using WT and recombinant β2GPI demonstrated excellent correlation at both 1:10 (Spearman's rho 0.70, P<0.001) and 1:100 dilution (Spearman rho 0.727, p<0.001) (Figure 1). Using the Wilcoxon test for paired samples, there was no significant difference between results of the ELISA using WT or recombinant β2GPI at 1:10 dilution (mean difference 4.26 ± 22.25, P<0.001) and a small difference at 1:100 dilution (mean difference 13.51 ±7.59, P <0.001) (Figure 2). Of the 32 patient samples, 6 were known positive for anti-β2GPI IgG (titer ≥ 20 GPL). Using a 90th percentile cutoff established using healthy volunteer samples, theELISA using recombinant β2GPI correctly identified 6/6 positive samples (sensitivity 100%). The ELISA using plasma-derived β2GPI correctly identified 5/6 positive samples (sensitivity 83.3%, specificity 84%).
Conclusion
Recombinant β2GPI can be produced in high yield by this novel method and purified with a single heparin chromatography step. It is recognized by anti-β2GPI Abat least as well as WT β2GPI. Further studies focused on site-directed mutagenesis of the intact molecule to optimize assays for detection of pathologic anti-β2GPI antibodies are underway.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal