Abstract
Introduction: There is an increased risk of venous thromboembolism (VTE) in patients with essential thrombocythemia (ET), however, what the absolute VTE risk is during pregnancy is largely unknown and is based on small cohort or registry studies. Recommendation for the use of low-molecular-weight heparin (LMWH) prophylaxis in the antepartum and/or postpartum setting is based on the absolute VTE and bleeding risk in other patient populations, for example, in women with inherited thrombophilia. A better estimate of absolute VTE risk will provide guidance for the use of LMWH in pregnant women with ET, as well as other treatments such as interferon.
Objective: The aim of our meta-analysis is to quantify the absolute risk of VTE with or without LMWH prophylaxis in women with ET during pregnancy and in the postpartum setting.
Methods: A systematic literature search was conducted on MEDLINE, EMBASE and EBM reviews. The primary outcome was VTE and the secondary outcomes were bleeding events and arterial thromboembolism (ATE). Studies were excluded if less than 5 pregnancies were reported, or if there was any duplication in publication, then only the most recent study was included. Using a standardized form, data was extracted independently by two investigators with disagreements resolved by consensus (L.S. and M.A.R.). Data on the use of LMWH, aspirin (ASA) and interferon was collected. Primary and secondary outcomes were reported per pregnancy in the antepartum and postpartum setting using pooled proportions, with 95% confidence intervals reported. Pregnancies ending in first trimester loss were excluded from the postpartum VTE analysis. Peripartum bleeding (delivery to 24 hours) was excluded. Placental abruptions were included as bleeding events.
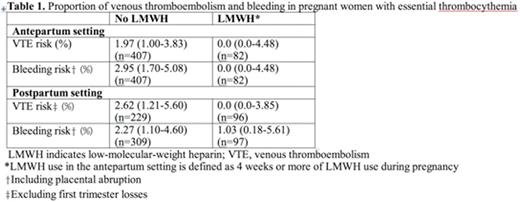
Results: There were 21 studies included that reported outcomes of interest in 504 patients and 756 pregnancies (1991-2016). Ten authors provided additional data. Among 756 pregnancies, there were 8 VTE events in the antepartum setting (1.06%, 95% CI, 0.54-2.07). Among 607 pregnancies (excluding first trimester losses), there were 8 VTE events in the postpartum setting (1.32%, 95% CI, 0.67-2.58). Of the 18 studies that reported VTE with respect to antepartum LMWH prophylaxis use, there were 0 antepartum VTE events among 82 pregnancies with LMWH use (0%, 95% CI, 0.0-4.48), and 8 antepartum VTE events among 407 pregnancies without LMWH use (1.97%, 95% CI, 1.00-3.83). Of the 14 studies that reported VTE with respect to postpartum LMWH prophylaxis use in pregnancies that excluded first trimester losses, there were 0 postpartum VTE events among 96 pregnancies with LMWH use (0%, 95% CI, 0.0-3.85), and 6 postpartum VTE events among 229 pregnancies without LMWH use (2.62%, 95% CI, 1.21-5.60) (Table 1).
There were 12 antepartum bleeds reported in 756 pregnancies (1.59%, 95% CI, 0.91-2.75), 9 of which were placental abruptions (1.19%, 95% CI, 0.63-2.25). There were 11 postpartum bleeds reported in 756 pregnancies (1.46%, 95% CI 0.81-2.59). Bleeding according to LMWH prophylaxis use in the antepartum and postpartum setting is presented in Table 1. Among 756 pregnancies, there were 4 possible ATE events described in the antepartum setting: transient visual loss after ASA was held for 1 week, an ocular TIA at 6 weeks gestation while on ASA, and 2 patients who described both visual disturbance and dizziness with unknown ASA use. No patient with possible ATE received LMWH prophylaxis.
Conclusion: Women with ET who are pregnant have an increased risk of VTE in both the antepartum and postpartum setting compared to the general pregnant population. The absolute risk of VTE in the antepartum and postpartum setting is not above a threshold where LMWH is clearly indicated or below a threshold where LMWH should be withheld. The role of thromboprophylaxis in this setting requires a shared decision-making process, taking into account patient values and preferences and the risks and benefits of LMWH.
Carrier:BMS: Research Funding; Leo Pharma: Research Funding. Robinson:Novartis: Honoraria; Pharmacosmos: Honoraria; Amgen: Honoraria. Rodger:Boehringer Ingelheim: Research Funding; Canadian Agency for Drugs and Technologies in Health: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal