Abstract
Canine immune thrombocytopenia (ITP), unlike mouse models of induced anti-platelet immunity, occurs spontaneously in non-inbred populations. Canine ITP demonstrates the same spectrum of bleeding severity and variable response to front-line therapy as the human disease counterpart. Pet dogs and their owners live in close contact in a shared environment with the same potential exposures to environmental triggers of an immune response. Moreover, the breed structure and large litter size of purebred dogs facilitate familial studies to uncover genetic risk factors of autoimmunity that are impossible to perform in human populations.
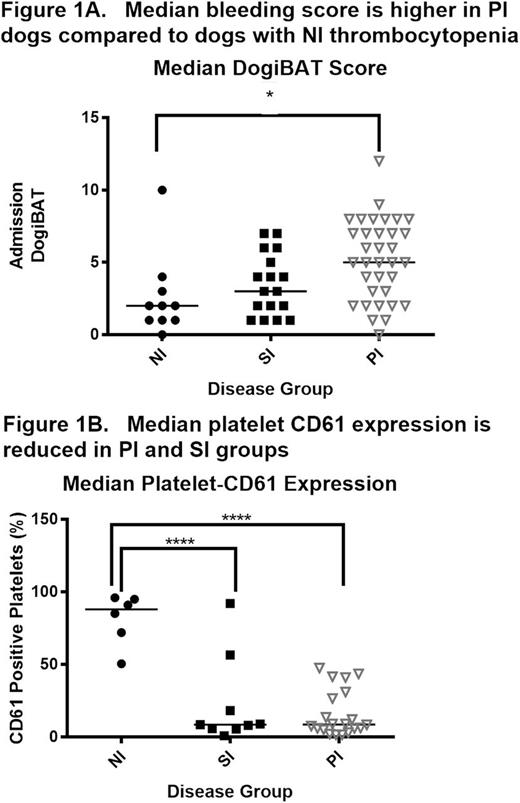
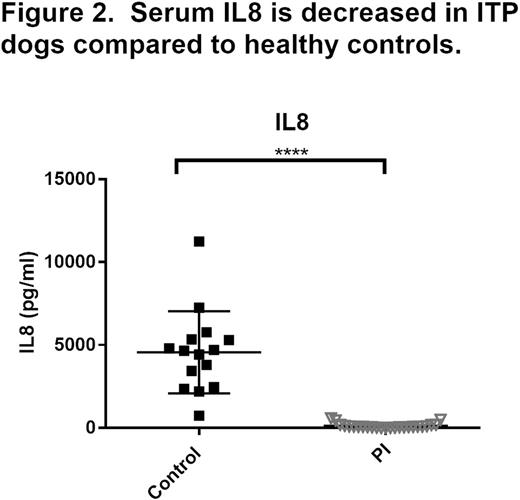
We performed a multi-institution cohort study of severe thrombocytopenia (defined as platelet count < 50,000/uL) in dogs to further develop the canine ITP model for comparative studies. At enrollment, bleeding severity was scored with a standardized bleeding assessment tool "DOGiBAT" based on the ITP bleeding scale, and samples were collected for routine clinicopathologic testing, immune profiling (cytokine analysis), and flow cytometric detection of platelet-bound antibodies (PSAIg). In the 61 dog sample set, immune-mediated platelet destruction was the most common cause of thrombocytopenia: 57% of cases had no inciting disease and were classified as primary immune (PI), 28% of cases were classified as secondary immune (SI) in association with lymphoid or myeloproliferative disease, drug exposure, or infectious disease, and the remaining 15% of cases had non-immune thrombocytopenia (NI) attributed to bone marrow aplasia or consumptive coagulopathy. The PI cases had a median age of 8 years, were 54% female, presented for petechiae and mucosal bleeding with high prevalence of PSAIg (76%) and had a 94% survival rate. These features are similar to the demographics, clinical findings, and low mortality of ITP in people. Among the significant differences between disease categories, PI dogs had higher DOGiBAT scores than NI dogs denoting more severe bleeding and both PI and SI cases had marked decreases in platelet CD61 expression compared to NI dogs (Figure 1, individual and group median values). Such aberrant platelet surface antigen expression has been described in childhood ITP. The surviving PI cases had a median duration of hospitalization of 4 days, with significant correlation between duration of hospital stay and admission DOGiBAT score (r2 = 0.18; p = 0.02). Serum cytokines and chemokines (Interferon (IFN) gamma, Interleukin (IL)-2, 6, 7, 8, 10, 15, 18, Canine orthologue of Chemokine C-X-C motif ligand 1 (KC), IFN-gamma-inducible protein-10, Monocyte chemoattractant protein-1, Granulocyte-macrophage colony-stimulating factor, and Tumor necrosis factor alpha) were measured with a multiplex assay (Milliplex CCYTOMAG-90K) in PI dogs and compared to those of healthy control dogs. Dogs with PI had significantly lower serum IL-8 compared to healthy control dogs (Figure 2). A negative correlation between platelet count and serum IL-8 has previously been reported in human ITP.
We also performed SNP genotyping for a genome-wide association study of ITP in purebred dogs. Initial analyses of 25 PI cases and 14 controls revealed over-representation of a dog leukocyte antigen (DLA) allele variant in PI dogs that was recently found to be associated with canine autoimmune disease in an independent study. Furthermore, we found a significant association between PI dogs and a novel locus on canine chromosome 1 (Figure 3, Manhattan plot).
Together, these preliminary phenotypic and genotypic studies reveal that canine ITP represents a new translational model to discover genetic loci and pathways associated with autoimmunity, biomarkers of primary and secondary immune targeted platelet destruction, and for proof of concept treatment trials utilizing novel therapeutic approaches.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal