Abstract
ESCORT-HU (European Sickle Cell Disease (SCD) COhoRT - HydroxyUrea) is a multicenter prospective non interventional study implemented in Europe, following the European Medical Agency's request to collect more information focused on long-term safety profile of hydroxycarbamide (HU) in SCD patients treated with HU. Primary endpoint of the study is to determine the frequency of adverse events (AEs) under HU treatment Secondary endpoints include the efficacy of HU in labeled indications (prevention of vaso-occlusive complications and of acute thoracic syndrome), frequency of hospitalizations due to SCD events and frequency of blood transfusions. Data collection on the reason for HU initiation permitted to identify in-label (in the therapeutic indication of the European marketing authorization, group 1, G1) and off-label (other therapeutic indication, group 2, G2) prescriptions of HU. Frequency of AEs (including not related to SCD and infections) was compared between adults and children, both in G1 and G2, with focus on the following subclasses: blood and lymphatic system disorders, fever, gastrointestinal disorders, infection, nervous system disorders, skin and subcutaneous tissue disorders and others.
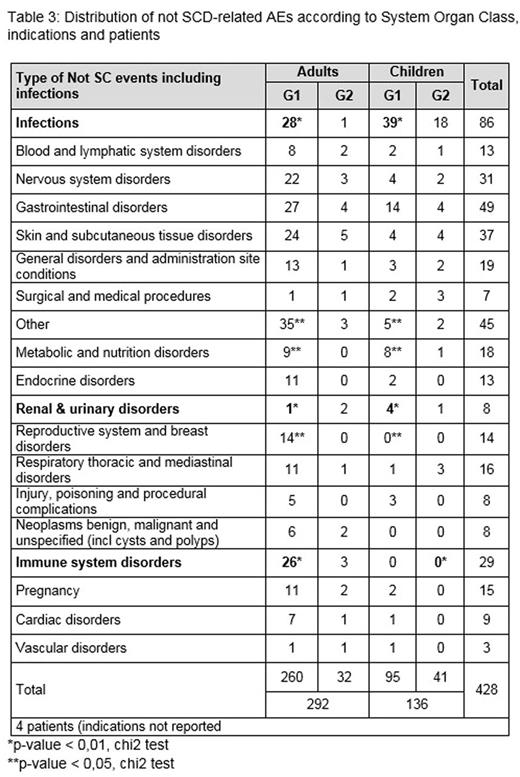
As of 6th June 2016 (cut-off date), a cohort of 1047 sickle cell patients have been enrolled in 3 European countries (Greece, 11.4%, Germany, 13.4%, and France, 75.2%). Of the 1047 patients, 845 (80.7%) have an in-label prescription (478 adults and 367 children), 170 (16.2%) an off-label prescription (61 adults and 109 children), and 32 reported unknown or no indication (Table 1a). As usually observed in any disease, the off-label use is more frequent in children compared to adults (p=0.01). Main reasons for off-label prescription were anemia (31%) (n=9 in adults and n=44 in children), abnormal Transcranial Doppler (TCD) values/cerebral vasculopathy (15%) (n=2 in adults and n=24 in children) and sickle cell organopathy including renal impairment (15%) (n=14 in adults and n=12 in children) (Table 1b). These three indications follow recommendations issued by European clinicians and are in accordance with the last version of US guidelines (JAMA 2014). Mean HU daily doses at initiation were respectively 18 mg/kg (G1) and 17.3 mg/kg (G2) in children, and 16.2 mg/kg (G1) and 13.5 mg/kg (G2) in adults. Not SCD-related AEs were respectively reported in 25% and 27.6% of patients in G1 and G2 groups (Table 2a). Focusing on the number of not-SCD related AE reported in each group, 355 AE have been reported in 211 patients in G1 and 73 AE have been reported in 47 patients in G2. Within G1 group, more AEs were reported in adults (30.8%) vs. children (17.4%) (p < 0.01) (Table 2b) but not in G2 group (NS) (Table 2c). When compared by AE type in G1, significant differences appeared between children and adults for the following AE subclasses (p < 0.01): infections more frequent in children as usually observed, renal & urinary disorders, immune system disorders more common in adults. Regarding neoplasms benign, immune system disorders, no malignancy has been reported, but mainly cysts (1 ovary, 2 breast), 1 renal tumor and 1 cutaneous lesion (histological types not provided), 1 other (location not reported) (Table 3). All AEs were more frequent in adults except infections.
In conclusion, the current ESCORT-HU data confirm the more frequent off-label use of HU in children, a similar frequency of patients reporting not SCD-related AE between the in-label and off-label groups despite a higher number of AE reported in the in-label group, and a significant difference between children and adults in frequency of AEs in the in-label group.
De Montalembert:Addmedica: Research Funding; Novartis: Research Funding, Speakers Bureau. Ribeil:Bluebirdbio: Consultancy; Addmedica: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal