Abstract
Background: Allogeneic transplantation (alloHSCT) is a potentially curative treatment option for patients (pts) with multiple myeloma (MM). Questions such as optimal donor source, role of graft versus host disease (GvHD), treatment-related mortality and patient selection still remain.
Methods: We report on 118 consecutive pts with MM who received an alloHSCT between November 2004 and September 2015 at the John Theurer Cancer Center. Pts received either a non-myeloablative (NMA), a reduced intensity (RIC) or a myeloablative (MA) regimen. The NMA regimens consisted of combinations of fludarabine (flu)/cyclophosphamide and low dose TBI. The RIC regimens consisted of flu/melphalan (mel) or flu/mel/bortezomib with the addition of low dose thymoglobulin (ATG) for unrelated donors (URD). The MA regimen was busulfan/mel, also with ATG for unrelated donors. All pts received at least one prior autograft. The consolidation group was defined as pts entering their allogeneic HSCT for consolidation after demonstrating a response (>/= PR) to their first autograft and without evidence of progression. The salvage group was defined as pts with < PR or with relapse after their first autograft.
Results: The median age was 54 years (range 35-66) and 48 (41%) pts were female. Seventy-five pts were in the consolidation group and 43 pts were in the salvage group. The hematopoietic cell donors were 67 related donors (RD) including 9 (8%) haplo-identical, and 51 URD including 17 (14%) mismatched donors. In our cohort, 10 (8%) pts received a MA, 72 (61%) received a RIC and 36 (30%) received a NMA regimen. The median follow-up for all surviving pts (n = 65) was 49.2 months (6.9-134.3). There were no significant differences between the RD and the URD cohorts except for lower age of unrelated donors (p<0.0001). Overall 40 (34%) pts achieved a CR, 35 (30%) pts a VGPR, 23 (19%) pts a PR and 10 (8%) pts had stable disease as their best response post transplantation. Sixty-two (52%) pts relapsed post transplantation, of whom 37 received DLI or underwent immune suppression withdrawal, with 15 pts (42%) responding to this intervention. Pts with an URD had a higher rate of all grade acute GvHD compared to pts with a RD, 52% versus 47% (p=0.042). There was no difference in grade III/IV acute GvHD. The cumulative incidence of all grade chronic GvHD was 54%, with no difference between recipients of RD or URD. The transplant-related mortality was 15.2% with no difference between the salvage and consolidation groups.
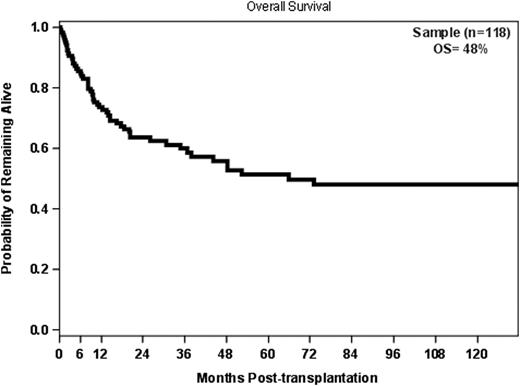
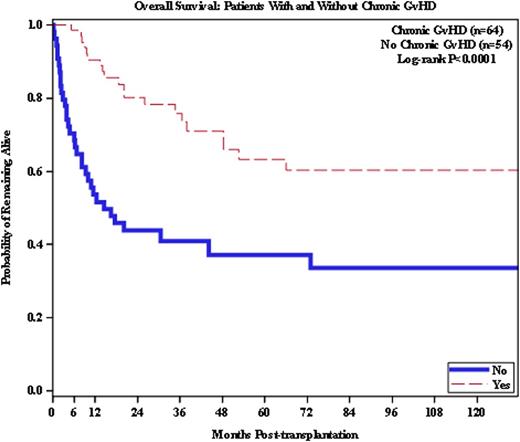
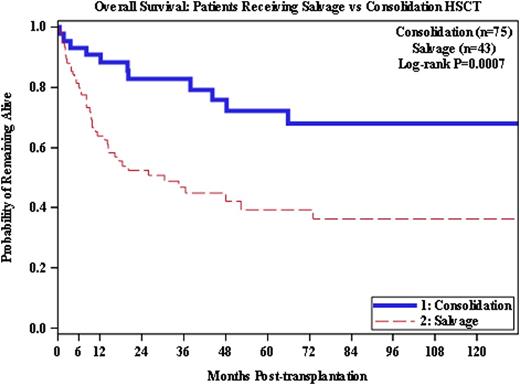
The overall survival (OS) for all 118 pts was 48% at 10 years. The OS at 10 years for the 43 pts who received alloHSCT as salvage was 36%, compared to 68% for the consolidation group (p=0.0007). In multivariate analysis, severe acute GvHD (grade III/IV), lack of chronic GvHD, and having two or more prior autologous HSCTs were significant predictors of decreased OS. Pts with severe acute GvHD had a greater mortality risk than pts without (p=0.0026), and having ≥ 2 prior auto HSCTs predicted for a higher risk of mortality (p=0.0447). Chronic GvHD was favorable, associated with a 36% improvement in overall survival (p=0.0008).
Conclusions: Long-term survival can be achieved in pts receiving allogeneic hematopoietic stem cell transplantation. This was observed in pts receiving alloHSCT for either salvage treatment or consolidation, with an acceptable transplant-related mortality. Donor source had no obvious effect on outcome. The development of chronic GvHD was significantly associated with improved survival (p=0.0008) supporting the importance of the graft-vs-myeloma effect.
Siegel:Novartis: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Merck: Honoraria; Celgene: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Vesole:Celgene: Speakers Bureau; Janssen: Speakers Bureau; Novartis: Speakers Bureau; Takeda: Speakers Bureau; Amgen: Speakers Bureau. Biran:Celgene: Speakers Bureau; Amgen: Speakers Bureau; Takeda: Speakers Bureau; Novartis: Speakers Bureau. Richter:Celgene: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Jannsen: Speakers Bureau. Skarbnik:Genentech: Speakers Bureau; Abbvie: Consultancy; Seattle Genetics: Speakers Bureau; Gilead Sciences: Speakers Bureau; Pharmacyclics: Consultancy. Goy:Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Other: Writing support, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genentech: Research Funding; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; Johnson & Johnson: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal