Abstract
<Introduction> Allogeneic hematopoietic stem cell transplantation (allo-SCT) is a potentially curative treatment for patients with non-remission myeloid malignancies. Although bone marrow (BM) or peripheral blood (PB) from HLA-matched related donor (MRD) have been the first choice of graft when available, true superiority of MRD over others can still be controversial especially for patients with non-remission myeloid malignancies. Cord blood (CB) has emerged as a promising alternative graft and its outcome has been encouraging over periods. Rapid availability is the advantage of MRD and CB over unrelated adult donor, which could fit to patients with non-remission myeloid malignancies who need urgent allo-SCT. So far, few studies are available comparing the outcomes of CB and MRD for adults with non-remission myeloid malignancies.
<Methods> We retrospectively reviewed the outcomes of patients with non-remission myeloid malignancies who underwent allo-SCT using CB or MRD at our institute from Jan. 2008 to Dec. 2015 consecutively. Patients who lacked MRD or were unable to find suitable unrelated donor within appropriate periods underwent CB transplantation. Patients who had a prior history of transplantation, were in poor performance status (ECOG PS 3 and greater), had active infections at the time of conditioning, were over 60 years, or received ATG as GVHD prophylaxis were excluded.
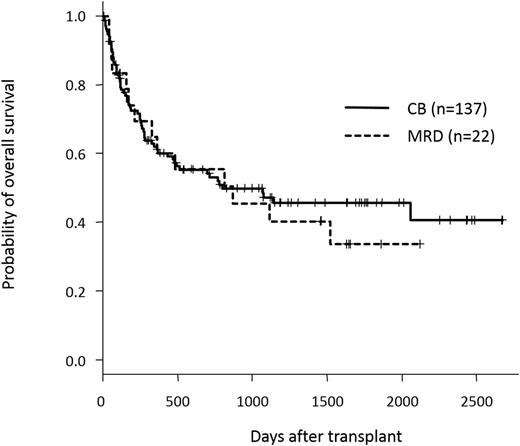
<Results> One hundred and fifty-nine patients were included in this study. One hundred and thirty-seven patients received single CB, whereas 21 receive PB from MRD and one received both PB and BM from the same MRD. Underlying diagnoses were AML (n=125), MDS-RAEB (n=20), CML (n=11), and MPN (n=3). All patients were not in remission at the start of conditioning regimens including primary induction failure (n=47), first relapse (REL) (n=37), second REL (n=4), and CML-BC/AP after TKI failure (n=11). Fifty-six patients who were diagnosed with AML with MRC or MDS were untreated or only received agents for blast control before transplantation. One hundred and twenty-nine patients were conditioned with MAC regimens, whereas 21 patients received RIC regimens. The median interval from diagnosis to transplant was 162 days (range, 34-2774 days). Recipients of CB and MRD were comparable in terms of median age (50 years in CB, 47 years in MRD), diagnosis, disease status, conditioning regimens, median interval from diagnosis to transplant, and year of transplant. CB recipients received more HLA-mismatched grafts and received a lower number of CD34+ cells, compared to MRD recipients. Median follow-up periods of survivors were 997 days for CB and 1459 days for MRD recipients, respectively. The overall (OS) and progression-free survival (PFS) showed no statistically significant differences between CB and MRD recipients; 3-year OSs of CB and MRD recipients were 47.2% (95% CI, 37.4-56.3%) and 45.5% (24.1-64.6%) (p=0.67), respectively (Figure 1), and 3-year-PFSs of CB and MRD recipients were 40.6% (31.4-49.6%) and 35.6% (15.7-56.2%) (p=0.3), respectively. Cumulative incidence of neutrophil engraftment was almost comparable between CB and MRD recipients (91% vs 100%, p=0.1), although CB recipients showed slower neutrophil recovery (median 21 (12-39) days vs 15 (11-39) days, p<0.01). Lower incidence of relapse was observed in CB recipients (31.5% vs 55.3% at 3 years, p=0.01), whereas CB recipients showed a trend to a higher NRM incidence relative to the MRD recipients, although not statistically significant (27.9% vs 9.1% at 3 years, p=0.19).
<Conclusions> Both transplant strategies have shown promising outcomes in patients with non-remission myeloid malignancies. Our analysis also shows that CB is not inferior to MRD as a source of hematopoietic stem cell grafts and may be associated with a lower relapse rate. CB could expand the possible donor pool for patients who need urgent allo-SCT and should be more considered as valuable grafts.
Izutsu:Abbvie: Research Funding; Gilead: Research Funding; Celgene: Research Funding; Janssen Pharmaceutical K.K.: Honoraria; Eisai: Honoraria; Kyowa Hakko Kirin: Honoraria; Chugai Pharmaceutical: Honoraria, Research Funding; Takeda Pharmaceutical: Honoraria; Mundipharma KK: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal