Abstract
Introduction: The impact of genomic alterations, such as mutations in ASXL1, on the risk of disease progression and leukemic transformation in patients with myelofibrosis (MF) is well established. Further, emerging data suggests that the number and type of mutations may impact response to therapies such as ruxolitinib or imetelstat. Allogeneic hematopoietic stem cell transplant (allo-HSCT) remains the only potentially curative treatment for MF patients. However, the impact of somatic mutations on overall survival (OS) and relapse-free survival (RFS) is poorly understood. Using next-generation sequencing of pre-transplant blood and bone marrow samples from a well clinically-annotated cohort of MF patients who underwent allo-HSCT, we sought to determine the impact of mutational burden on outcomes.
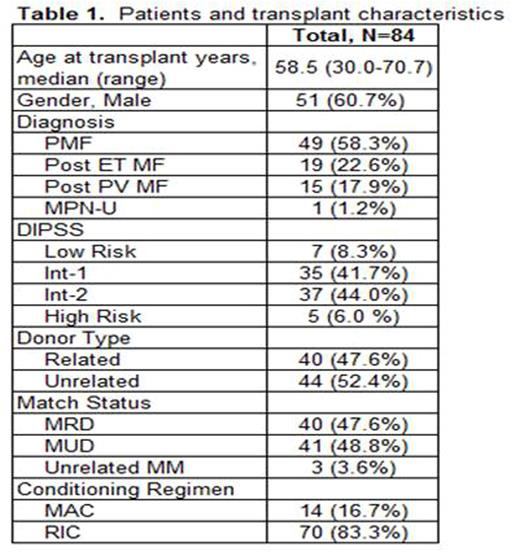
Methods: A multicenter retrospective analysis of a cohort of 84 patients was carried out. This included 52 patients treated on the MPD-RC 101 prospective study (NCT00572897), 18 patients treated at Prince Margaret Hospital, and 14 patients treated at Memorial Sloan Kettering Cancer Center. Patient and transplant characteristics are displayed in Table 1. DNA was extracted from pre-transplant bone marrow aspirate samples or peripheral blood samples. High-throughput sequencing of a panel of genes was performed. Average coverage of 829x (standard deviation of ±130) was obtained. Mutect was utilized to call single point variants (comparing our samples to a pool of normal samples) and PINDEL was used to call short insertions and deletions. We excluded all mutations present in at least one database of known non-somatic variants (DBSNP and 1000 genomes) and absent from COSMIC. Univariate Cox regression and Kaplan-Meier graphics were used to investigate the association of patient, transplant, and disease characteristics with OS and RFS.
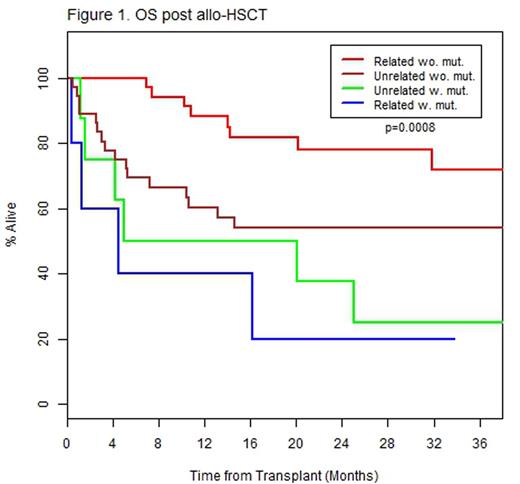
Results: JAK2V617F was the most frequent mutation detected in 41(48.8%) patients (Table 2). Eighteen patients (21.4%) had triple negative disease (negative for JAK2, MPL, and CALR mutations). Univariate analysis included the following: patient characteristics (age, gender), transplant characteristics (related vs. unrelated donor, matched vs. mismatched donor and myeloablative vs. reduced intensity conditioning) and disease characteristics (DIPSS and presence of mutations). Decreased OS was associated with unrelated donor status (HR 2.09, 95% CI: 1.03-4.23, p=0.04), reduced intensity conditioning (HR 4.21, 95% CI: 1.01-17.59, p=0.049), triple negative disease (HR 2.09, 95% CI: 1.02-4.30, p=0.04), and presence of U2AF1 (HR 2.53, 95% CI: 1.10-5.81, p=0.03) or SUZ12 mutations (HR 3.92, 95% CI: 1.19-12.21, p=0.02). Decreased RFS was associated with unrelated donor status (HR 2.27, 95% CI: 1.16-4.45, p=0.02), and the presence of SUZ12 mutation (HR 6.97, 95% CI: 2.37-20.49, p<0.001). A descriptive decrease in RFS in patients with U2AF1 (HR 2.15, 95% CI: 0.94-4.88, p=0.07) was observed but did not reach statistical significance. Importantly, mutations previously reported to be associated with reduced OS and RFS in the non-transplant setting, such as ASXL1, EZH2, IDH1/2, and SRSF2, were not associated with poorer outcomes in this analysis in transplanted patients. In an exploratory multivariate analysis including donor type (related vs. unrelated) and presence of U2AF1 and SUZ12 mutations, there was a significantly reduced OS and RFS in patients who harbor these mutations regardless of donor type (OS: HR 5.30, 95% CI: 2.08-13.47, p<0.001; RFS: HR 5.49, 95% CI: 2.27-13.30, p<0.001). In patients without the above mutations, having an unrelated donor was associated with worse OS (HR 2.55, 95% CI: 1.09-5.96, p=0.03) and RFS (HR 2.61, 95% CI: 1.17-5.83, p=0.02, Figure 1).
Conclusions: Our analysis demonstrates that mutations previously associated with poor prognosis in MF, such as ASXL1, do not appear to confer a worsened prognosis in patients undergoing allo-HSCT, suggesting transplant may be able to overcome the impact of these mutations. However, mutations in SUZ12 and U2AF1 are associated with reduced OS in univariate and multivariate analysis (together with donor type). Further studies with larger cohorts of patients are indicated to validate these findings, and to elucidate the impact of these mutations on disease biology.
Rampal:Incye and CTI: Consultancy. Mascarenhas:Janssen: Research Funding; CTi Biopharma: Research Funding; Promedior: Research Funding; Merk: Research Funding; Incyte: Research Funding. Mesa:Galena: Consultancy; Gilead: Research Funding; Promedior: Research Funding; Incyte: Research Funding; CTI Biopharma: Research Funding; Celgene: Research Funding; Ariad: Consultancy; Novartis: Consultancy. Gupta:Novartis: Consultancy, Honoraria, Research Funding; Incyte Corporation: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal