Abstract
*The 2 first authors contributed equally to the work.
Background. The best post-remission treatment for younger acute myeloid leukemia (AML) patients has remained controversial (Cornelissen JJ &Blaise D, Blood 2016, 127, 62-70) and there is paucity of studies comparing intensive chemotherapy to autologous (auto) or allogeneic (allo) bone marrow transplantation (BMT).
Aim. The main objective of this study was to provide a long-term follow-up evaluation of patients previously enrolled in the pivotal EORTC/GIMEMA AML-8A (Zittoun et al., New England Journal of Medicine 1995, 332, 217-223).
Methods. The EORTC/GIMEMA AML-8A prospectively assessed the impact of 3 post-remission treatments on disease-free survival (DFS) and overall survival (OS) in younger (<60 years of age) AML patients who reached a complete remission (CR) after induction with cytarabine and daunorubicin. All patients received a consolidation chemotherapy course (ICT1) including intermediate-dose cytarabine and amsacrine. Patients who had an HLA-identical sibling donor were then allocated to allo-BMT while remaining patients, at the start of ICT1, were randomized to receive auto-BMT or a second course of intensive chemotherapy consisting of high-dose cytarabine and daunorubicin (ICT2 group) after recovery of ICT1. At the time of the publication of the results (Zittoun et al., New England Journal of Medicine 1995, 332, 217-223), the median follow-up from inclusion was 3.3 years.
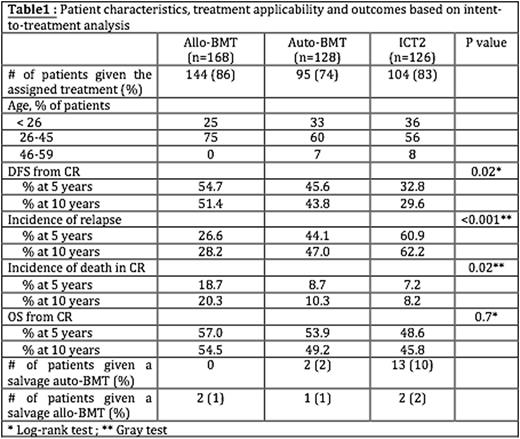
Results. In the current report, median follow-up was 11.1 (range, 0-28) years. For the whole population (n=422), the 5-, 10 and 15-year OS rates from inclusion were 35%, 32% and 31%, respectively. After the completion of ICT1, 168 patients were allocated to theallo-BMT arm, while 254 patients were randomized to auto-BMT (n=128) or ICT2 (n=126). DFS from CR was longer afterallo-BMT than auto-BMT and DFS from CR was longer after auto-BMT than ICT2, due to a lower relapse incidence (P<0.001). Details are provided in table 1. Patients randomized to the auto-BMT arm had still a longer DFS from CR than patients in the ICT2 group (P=0.11) due to a lower incidence of relapse (P=0.047). Regarding OS, the differences between the 3 groups was no longer significant. In the ICT2, the improved outcome regarding OS as compared to DFS was due to a higher proportion of ICT2 patients who received a salvage auto-BMT.
Conclusions. This long-term follow-up of the EORTC/GIMEMA AML-8A study confirms a better DFS with allo-BMT or auto-BMT when compared to ICT2 for AML patients in first CR. Further, this long-term follow-up study revealed that the vast majority of patients alive in first CR at 5-year remains disease-free survivors 5 years later. Although indications of allogeneic hematopoietic stem cell transplantation (allo-HCT) are nowadays largely driven by cytogenetic/molecular AML profile, long-term results of AML8A study demonstrate that auto-BMT remained superior to ICT2 in younger AML patients not candidate for an allo-HSCT.
Efficace:TEVA: Consultancy, Research Funding; Seattle Genetics: Consultancy; Bristol Myers Squibb: Consultancy; Lundbeck: Research Funding. Martinelli:Celgene: Consultancy, Speakers Bureau; BMS: Speakers Bureau; Novartis: Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Genentech: Consultancy; Roche: Consultancy, Speakers Bureau; Ariad: Consultancy, Speakers Bureau; MSD: Consultancy; Pfizer: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal