Abstract
Introduction. Graft failure/rejection (GF/R) is a significant complication following HCT in non- malignant diseases. The risk of GF/R in patients with thalassemia is particularly high as a consequence of alloimmunization related to transfusion exposures and hyperproliferative marrow. Although the GF/R rate after myeloablative HLA-matched sibling transplants for thalassemia has been reduced, its incidence still remains high following alternative donor transplantation. Typically, GF/R after HCT for thalassemia is accompanied by autologous hematopoietic recovery, but occasionally patients develop GF with prolonged marrow aplasia. Given great variability in conditioning regimens and outcomes after second HCT for GF/R, it is important to develop a uniform treatment approach to patients receiving second transplantation for GF/R with autologous recovery.
Methods. We report on 21 consecutive patients with median age of 9 years (range, 4-24 years) with thalassemia who underwent a second HCT for GF/R with autologous recovery. Ten patients had primary and 11 patients secondary GF/R following the first BMT. Median time to second transplant was 41 months (range, 8- 204). Treatment protocol consisted of preconditioning cytoreduction/immunosuppression with hydroxyurea (30 mg/kg/day) and azathioprine (3 mg/kg/day) from day −45 pre-transplant, and fludarabine (30 mg/m2/day) from day −16 through day −12. Conditioning regimen included oral or intravenous (i.v.) busulfan, thiotepa 10 mg/kg/day, cyclophosphamide 200 mg/kg total dose, and thymoglobulin 12.5-10 mg/kg total dose. Cyclosporine, methylprednisolone and methotrexate were given for GVHD prophylaxis. All but 4 patients received second HCT from the same donor. Thirteen patients received bone marrow (BM), and 8 patients PBSC. One patient was in class 1, 7 patients in class 2, and 13 patients in class 3 of risk. Most patients had moderately severe iron overload with median serum ferritin of 2692 ng/ml (range, 500-10126 ng/ml). The median liver fibrosis score 1 (range 0-5).
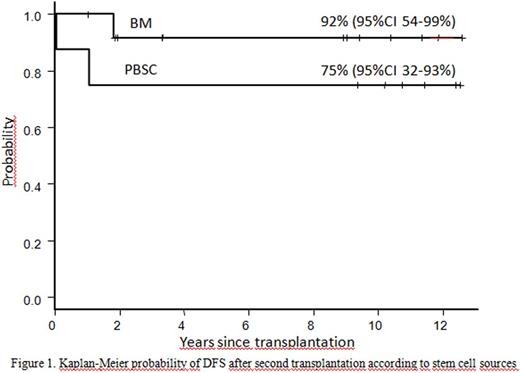
Results. All but 1 patient achieved sustained engraftment with a 5 year overall and disease-free survival (DFS) of 85% (95% CI, 59-95%). DFS was 92% (95% CI, 54-99%) in patients receiving BM and 75% (95% CI, 32-93%) in those given PBSC, although the difference was not statistically significant (p=0.27) (Figure 1).
One patient had primary GF with the cumulative incidence of 5% (95% CI, 0-13%). The incidence of grade 2-4 acute GVHD was 50% (95% CI, 0-75%) in the PBSC and 8 % (95% CI, 0-24%) in the BM group (p=0.025). Respective incidences of moderate to severe chronic GVHD were 43% (95% CI, 0-70%) and 0% (p=0.012). The high incidence of both acute and chronic GVHD after PBSC transplantation prompted us to abandon its use for second transplantation since 2006. At the time of survival analysis, 18 patients were alive, with median follow-up duration of 10 years (range, 1.2-12.8 years). At present all patients are off immunosuppressive medication. There were 3 deaths due to pneumonia, postsplenectomy sepsis and rejection with cerebral bleeding. The most frequently observed toxicities were grade 2 elevations in AST and ALT, followed by grade 2 oral mucositis. Few patients experienced grade 3 liver and gut toxicities. None of the patients developed liver VOD. Three patients had pneumonia, and 4 patients developed grade 2-3 late onset BK virus -related hemorrhagic cystitis. None of the patients developed lymphoproliferative disorder.
Conclusions. We demonstrate the excellent DFS in patients with thalassemia treated with second transplantation for GF/R following the first graft. Importantly, the intensified treatment protocol was not associated with increased nonhematological toxicity, even though most patients suffer from pre-existing organ damage due to iron overload and/or hepatitis. This study clearly showed that the same donor could be successfully used for second transplantation in these patients. Due to significantly high incidence of both acute and chronic GVHD after PBSC, bone marrow graft is preferred source for second transplantation in patients with thalassemia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal