Abstract
Introduction: Monocyte recovery following allogeneic hematopoietic cell transplantation (allo-HCT) has previously been correlated with improved overall survival (OS). Three distinct monocyte subpopulations have been identified based on CD14 and CD16 surface expression, including: CD14+CD16++ (non-classical), CD14+CD16+ (intermediate), and CD14++CD16- (classical). Among these subpopulations, non-classical monocytes have greater capacity to produce inflammatory cytokines, whereas classical monocytes have phagocytic activity, are less inflammatory and produce counter regulatory cytokines (i.e., IL-10), and intermediate monocytes are a transitionary population sharing features of both non-classical and classical monocytes. To date, there has not been a direct comparison between stem cell source and monocyte recovery, nor has there been an analysis of monocyte subpopulation recovery and HCT outcomes. We hypothesized that recovery of monocytes and their subpopulations would vary based on stem cell source and that recovery of the monocyte subpopulations would be associated with clinical outcomes.
Methods: This analysis included individuals who underwent a first allo-HCT at the University of Minnesota between 2010-2014 and who enrolled onto an institutional immune reconstitution protocol. Absolute monocyte count (AMC), as well as the absolute counts of non-classical, intermediate and classical monocyte subpopulations were assessed at days 28, 60, 100 and 365 post-HCT. Optimal cut points of each monocyte subpopulation at day 28 were calculated using the Contal and OQuigley method and were used for analysis. This time point was selected because the outcomes of interest were unlikely to have occurred prior to this time. Individuals who experienced acute graft versus host disease (aGVHD) prior to day 28 were excluded from the aGVHD analysis (n=39). Patient demographics, disease and treatment characteristics were included in univariable analyses to determine associations between monocyte subpopulations and transplant outcomes, including 2-year OS, 1-year transplant related mortality (TRM), 2-year disease free survival (DFS), 2-year relapse, 180-day aGVHD and chronic GVHD. Factors with P-values <0.15 were included in multivariable models.
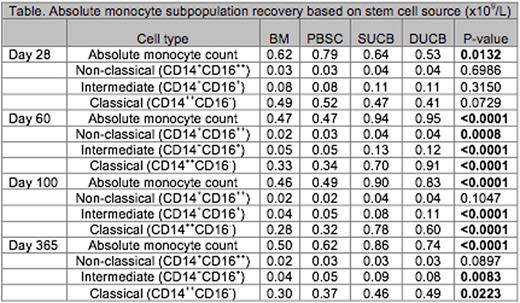
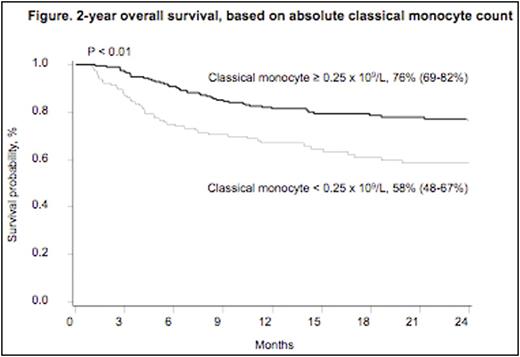
Results: Among 318 individuals with available day 28 data, median age at HCT was 38 years, and median follow up was 531 days. Conditioning was myeloablative for 45% and reduced intensity for 55%. Stem cell source was: BM (n=80), PBSC (n=79), single UCB (SUCB) (n=54) and double UCB (DUCB) (n=105). Although monocyte subpopulation recovery did not differ across stem cell sources at day 28, AMC, classical and intermediate monocyte recovery was greater among UCB recipients compared to other stem cell sources at days 60, 100 and 365 (Table). In multivariable models, after controlling for each monocyte subpopulation, stem cell source, disease and treatment characteristics, an absolute classical monocyte count of >/= 0.25x 109/L at day 28 was associated with improved OS (HR=0.33, 95% CI 0.12-0.86, P=0.02) (Figure) and DFS (HR=0.36, 95% CI 0.15-0.85, P=0.02). Absolute numbers of non-classical monocytes >/=0.02 x 109/L at day 28 was associated with decreased rates of grade II-IV aGVHD (HR=0.58, 95% CI 0.34-0.99, P=0.04) and decreased cGVHD (HR=0.48, 95% CI 0.26-0.90, P=0.02). None of the day 28 monocyte subpopulation counts were associated with TRM or relapse.
Conclusions: Monocyte recovery differs among stem cell sources. UCB recipients experience more robust monocyte recovery, specifically within the intermediate and classical monocyte subpopulations, at day 60 and at all measured time points beyond, compared to other stem cell sources. Early monocyte recovery is associated with HCT outcomes. Increased classical monocyte count at day 28 is independently associated with improved OS and DFS. Paradoxically, despite their inflammatory properties, increased non-classical monocyte count predicts decreased acute and chronic GVHD within this large series of patients. If reproduced, these findings could allow for enhanced outcome prediction models and could lead to novel interventions to improve transplant outcomes.
Cooley:Fate Therapeutics: Research Funding. Miller:Oxis Biotech Scientific Advisory Board: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal