Abstract
Background: CB transplantation (CBT) can be associated with early post-transplant complications resulting in prolonged hospitalization, but this disadvantage may be offset by the later benefits of low rates of chronic GVHD & relapse, & high rates of long-term immune recovery. Our center is investigating CBT after a novel intermediate intensity, reduced toxicity myeloablative conditioning regimen in an effort to reduce early complications/ toxicities while maximizing disease control. However, the determinants of both progression-free survival (PFS) & the complications that compromise the time spent outpatient & progression-free after this therapy are not established.
Methods: We analyzed factors contributing to 2-year PFS & the composite endpoint of being both outpatient & progression-free by post-transplant time period in adult recipients of intermediate intensity CBT. Eligible patients (pts) were 1st allograft recipients ≤ 65 years conditioned with cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2, thiotepa 5-10 mg/kg, 400 cGy TBI for the treatment of acute leukemia/ MDS/ MPD (≤ 10% blasts pre-CBT), B-cell NHL or HL. GVHD prophylaxis was with CSA/ MMF (no ATG).
Results: Pts [n = 110, 55% non-European, 62% CMV seropositive, median age 51 years (range 18-65)] had diagnoses of acute leukemia (83), MDS/MPD (11), B-cell NHL (14) & HL (2). 109 received double & 1 single unit grafts [median donor recipient 8-allele HLA-match 5/8 (range 2-8); median infused CD34+ dose 0.95 x 105/kg/unit (range 0.17-3.72)]. 96% of pts engrafted. Day 180 incidence of grades II-IV & III-IV aGVHD was 75% (95%CI: 66-83) & 21% (95%CI: 14-29), respectively; 8% (95%CI: 4-15) of pts had cGVHD by 1 year. Day 180 TRM incidence was 15% (95%CI: 8-22), & 10% (95%CI: 5-17) of pts relapsed by 2 years post-CBT. With a median survivor follow-up of 2.7 years (range 6 months-8.5 years), the 2-year OS is 72% (95%CI: 64-81) & PFS is 69% (95%CI: 61-79). Revised disease risk index (rDRI) & age adjusted hematopoietic cell transplant-comorbidity index (aaHCT-CI) were significant determinants of 2-year PFS (Figure 1), whereas pt age (< 51 vs ≥ 51 years), CMV serostatus, European vs non-European ancestry, dominant unit-recipient HLA-match (< 5/8 vs ≥ 5/8) & infused CD34+ cell dose (< 0.95 vs ≥ 0.95) were not. In multivariate analysis, only aaHCT-CI was significant [HR 2.15 (95%CI: 1.01-4.59) if aaHCT-CI ≥ 3 (p = 0.047)]. The HR if high-very high rDRI was 2.03 (95%CI: 0.92-4.48) (p = 0.079).
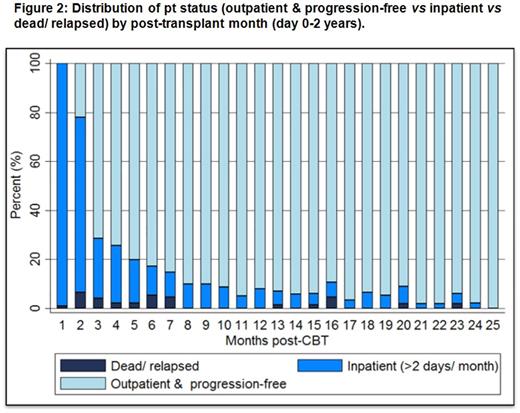
We then analyzed if recipient & graft factors were associated with the endpoint of being outpatient & progression-free by time period post-CBT. The median initial length of stay was 37 days (range 14-77) post-CBT. 101/110 (92%) pts survived to first discharge. Within the first 180 days, 60/101 (59%) of these pts were re-admitted for transplant-related complications at least once [111 readmissions, median 1/pt (range 1-9), & most commonly due to infection or aGVHD (± infection)]. However, when the distribution of pts who were outpatient/ progression-free vs inpatient vs dead/ relapsed was analyzed by post-transplant month over the first 2 years (Figure 2), high proportions of pts are outpatient & progression-free after 6 months post-CBT. Finally, we found that low-intermediate rDRI, 0-2 aaHCT-CI & CMV seronegativity were associated with a higher mean number of outpatient progression-free days in the first 6 months (Table), and only aaHCT-CI was significant through-out the 2 years post-CBT. Age, ancestry & graft variables were not significant at any time. In multivariate analysis for days 0-180, only aaHCT-CI was significant (p = 0.015).
Conclusions: The 2-year PFS in this adult pt population (median age 51 years) is very promising after Cy/Flu/Thio/TBI intermediate intensity CBT. Not only is the incidence of late TRM and relapse low, the burden of hospitalization is low after 6 months resulting in high rates of being outpatient and progression-free long-term. Patients with low aaHCT-CI scores do especially well and PFS with quality of life comparisons of this pt population as compared to allografting with other stem cell sources is indicated.Nonetheless, new strategies are required to reduce early post-CBT complications, and further investigation is required to understand which comorbidities specifically dictate increased risk for complications and mortality, as well as how to improve CBT safety in pts with high aaHCT-CI.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal