Abstract
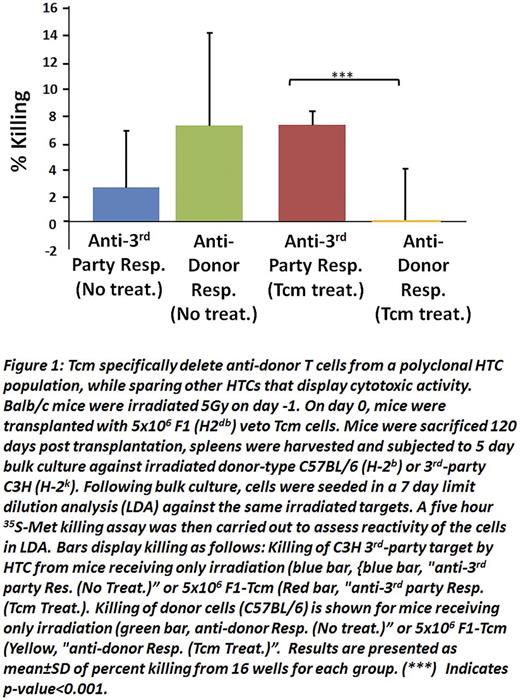
We recently demonstrated that mouse anti-3rd party central memory CD8 veto cells (Tcm) enable engraftment of fully mis-matched T cell depleted bone marrow (TDBM) in sublethally irradiated (5.5 Gy TBI) recipients (Eran et al. Blood 2013). Here we found that Tcm generated from (host x donor)F1 or fully allogeneic donors were able to engraft and sustain their presence in such sub-lethally irradiated mice in the absence of TDBM transplants. Limiting dilution analysis revealed that the reactivity of host T cells (HTC) against donor spleen cells was significantly diminished in the chimeric mice treated with Tcm, whereas reactivity against 3rd party was comparable betweenTcm treated and non-treated mice (Fig. 1). This specific immune tolerance indicated that vetoTcm could potentially enable the use of 'off-the-shelf' allogeneic CAR T cells or any other genetically modified T cells from the same donor.
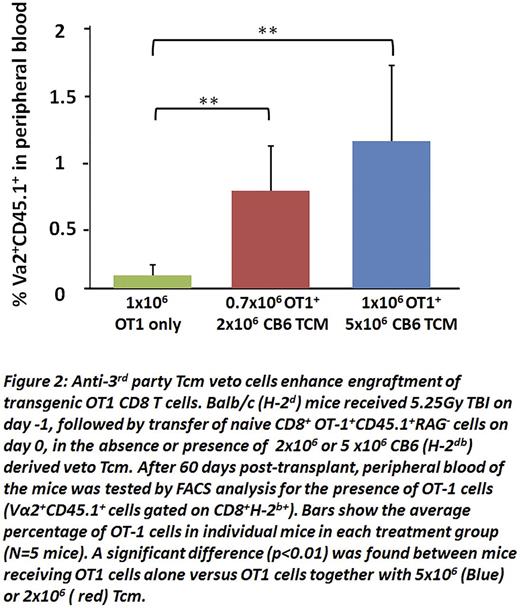
To further investigate this possibility, we tested the ability of Tcmveto cells to enable engraftment of CD8 T cells from OT1 mice, expressing a transgenic T cell receptor (TCR) directed againstovalbumin (OVA) residues 257-264 in the context of H2Kb MHC-I. To that end, 1x106 OT1 CD8 T cells (H2b) were infused into Balb/c (H2Dd) recipients sublethally irradiated with 5.25 Gy TBI in the presence or absence of different doses of veto Tcm. As shown in Fig. 2, when tested at 2 months post-transplant, OT1 cells could not be detected in the peripheral blood of untreated recipient mice, while they were readily detectable in mice receiving 2x106 Tcm (0.78±0.36), reaching a higher level (1.18± 0.61) upon infusion of 5x106Tcm.
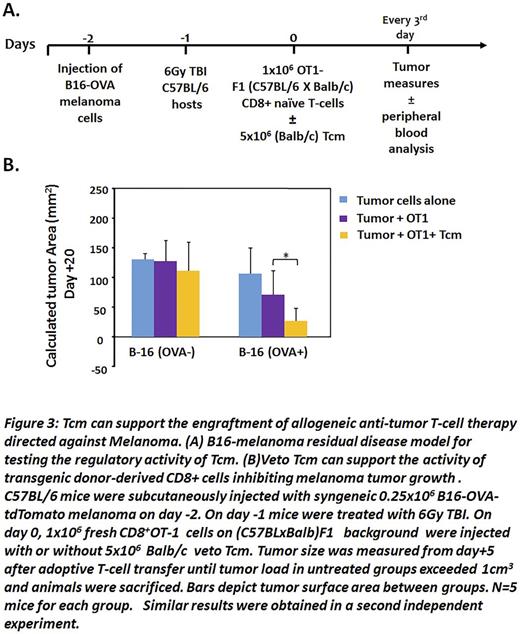
To evaluate the functionality of the engrafted OT1 T cells in the chimeric mice, we developed and calibrated a new murine model, using a melanoma B16-cell line of C57BL background that expresses the ovalbumin peptide and the tdTomatomarker (B16-OVA-tdTomato) (Fig 3A). In this model, we tried to mimic a state of minimal residual disease by injecting a small number of B16-OVAtdTomato melanoma cells (0.25x106) into syngeneic C57BL mice; we then treated the mice with sublethal irradiation (6Gy TBI) to simulate treatment of the tumor, but also to clear out space for the T-cell adoptive transfer. Finally, we injected 1x106 naïve OT-1 cells generated on a (Balb x-OT1-CD45.1+RAG2-) F1 background in the presence or absence of Balb/c veto Tcm (5x106), and followed the development of the tumor. We found thatBalb/c vetoTcm prolonged the engraftment of the transgenic donor-derived F1 OT1 CD8 T cells inBalb/c recipients, thereby enabling significantly improved control of tumor growth when tested at day 20 post-transplant ( p<0.05). In contrast non OVA-expressing tumor cells grew at the same rate in all treatment groups, thereby demonstrating the specificity of the anti-tumor effect (Fig.3B).
Previous studies demonstrated that Tcmare depleted of GVH reactivity by virtue of their culture with 3rd party stimulators under cytokine starvation. Our present results demonstrate that suchTcm veto cells can pave the way for functional engraftment of CD8 T cells with genetically modified specificity. Taken together, these results provide a proof of concept for the potential clinical use of such veto cells to enable therapy with 'off-the-shelf ' allogeneic CAR T cells. The relative ease of growing humanTcm in large numbers over a short period of time (9-12 days) suggests that it could be possible to harvest byleukapheresis from a single donor, a sufficient number of cells, to support CAR therapy for more than 50 allogeneic recipients.
* N.O.G and R.G equally contributed
Or Geva:Yeda LTD: Patents & Royalties. Gidron:Yeda LTD: Patents & Royalties. Reisner:Cell Source LTD: Consultancy, Equity Ownership, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal