Abstract
Chimeric Antigen Receptors (CARs)-redirected T lymphocytes are a promising novel immunotherapeutic approach, nowadays object of accurate preclinical evaluation also for the treatment of Acute Myeloid Leukemia (AML). In this context, we recently developed a CAR against CD123 (IL-3 receptor α subunit), a tumor associated antigen over-expressed on AML blasts and leukemic stem cells. However, the potential recognition by anti-CD123 CAR-T cells of low CD123 positive healthy tissues, i.e. endothelium and monocytes, through the known "on-target-off-organ" effect, warrants careful preclinical investigation for a safe employment of this approach in the clinic. Therefore, in search for an optimization of this strategy, we evaluated the effect of several variables implicated in the CAR design, known to modulate CAR T-cell functional profiles in a context-dependent manner, such as CAR binding affinity, CAR expression and target antigen density.
Computational structural biology tools have been exploited to design rational mutations in the anti-CD123 CAR antigen binding domain that altered CAR expression and CAR binding affinity. To this aim, Cytokine-Induced Killer (CIK) effector cells have been genetically modified with four Chimeric Affinity Mutants (CAMs), represented by CAM-H1 (High Affinity 1), CAM-H2, which maintain the same binding affinity of the wild type (wt) anti-CD123 CAR (10-9 M), CAM-M (Medium affinity) and CAM-L (Low affinity), displaying a 10- and 100-fold affinity reduction, respectively.
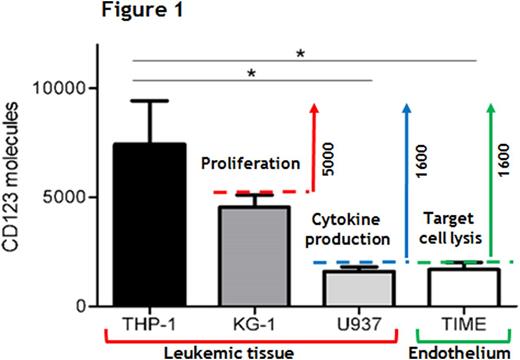
The functional characterization of CAM-CIK cells revealed both their specificity and effectiveness in early cytotoxic responses against both the high CD123+ THP-1 AML cell line and primary AML cells, reaching more than 70% of killing activity as the wt CAR-CIK cells. However, in this context, among all the variables analyzed, a CAR expression level up to ≈40% proved to be necessary for inducing effective later effector functions towards a high CD123+target, such as proliferation and cytokine production. To mimic the CD123 expression on leukemic and healthy tissues, a panel of cell lines with different surface levels of CD123 was introduced. In particular, a number of ≈1600 CD123 molecules was sufficient to induce a good cytotoxic response by all the CARs tested, with the CAM-L being the less powerful against both the AML U937 cell line and healthy TIME endothelial cell line, sharing the same target antigen density, and allowing for the identification of ≈1600 CD123 molecules as the "lytic threshold" in our setting. At the same time, this antigen density was not enough to determine a good proliferative capability for all the CAR-CIK cells tested, which instead occurred with leukemic cells expressing ≈5000 or more CD123 molecules. Cytokine release induced by all CAM-CIK cells was instead directly proportional to target antigen density, starting from ≈1600 molecules/cell, indicating that both these "activation thresholds" depend on different target antigen densities for being respectively triggered. As a matter of fact, a proportional CAR down-modulation in response to increasing target antigen densities was observed, implying this mechanism as one of the principal determinant for CAR-CIK cell related functional outcomes, independently of CAR binding affinity. Furthermore, with the aim of evaluating the response of CAM-CIK cells against the endothelium in a more physiological context, we set up a co-culture of Matrigel-embedded endothelial cells and CIK cells. The same and limited impairment of vessel formation was observed, with no differences encountered between un-manipulated and CAR-redirected CIK cells, implying that anti-CD123 CAR CIK cells are not activated more than the un-manipulated counterpart towards this target, independently of CAR binding affinity.
In conclusion, we were able to define both "lytic" and "activation" antigen thresholds (Figure 1), showing that whereas the early T-cell cytotoxic activity is not affected either by CAR expression or CAR affinity tuning, later effector functions are impaired by low CAR expression. Moreover, a promising balance in the efficacy and safety profiles of CAR-CIK cells was observed in the lowest affinity mutant in response to targets with low CD123 densities. Overall, the full dissection of all these variables offers additional knowledge for the proper design of a suitable anti-CD123 CAR for the treatment of AML.
Biondi:Cellgene: Other: Advisory Board; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal