Abstract
Adoptive T cell therapy (ATT) is a promising option for the treatment of solid cancers. However, various defense mechanisms acquired by the tumor during evolution prevent transferred T cells (TC) to unfold their full potential. A combination of ATT with accessory therapeutic approaches including checkpoint inhibition and targeted therapy could lift TC inhibition and efficiently shift the immune balance towards tumor rejection. An in-vivo analysis of the impact of combination strategies on the outcome of ATT would greatly enhance the search for an optimal accessory to ATT therapy.
We generated the transgenic mouse line BLITC (bioluminescence imaging of T cells) expressing an NFAT (nuclear factor of activated T cell)-dependent Click-beetle luciferase (Na et. al, 2010) and a constitutive Renilla Luciferase, allowing us to monitor migration and activation of transferred TCs in vivo. In order to analyze crucial ATT parameters in a clinically relevant tumor model, BLITC mice were crossed to the two HY-TCR transgenic mice Marilyn (CD4: H-2Ab-Dby) and MataHari (CD8: H-2Db-Uty) to generate TCs that could be monitored for in-vivo infiltration, local activation and rejection of established (> 0,5 cm x 0,5 cm / ≥10 days growth) H-Y expressing MB49 tumors. In order to better reflect the clinical situation, we lymphodepleted tumor-bearing immunocompetent albino B6 mice with fludarabine (FLu) and/or cyclophosphamide (CTX) prior to ATT. Transferred TCs were FACSorted and injected after an optional culture expansion phase.
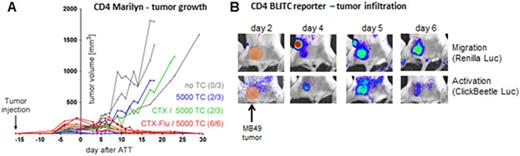
As shown before for freshly injected tumor cells (Perez-Diez, 2007), we observed a superior response of tumor-antigen specific CD4+ TCs compared to CD8+ TCs against established tumors. Whereas 5*106 CD8+ T cells hardly attenuated tumor growth, even as few as 5000 H-Y TCR-transgenic CD4+ T cells rejected tumors in most mice, depending on the lymphodepleting treatment (Figure A - remission rates in parentheses). Tumor infiltration and activation of adoptively transferred TCs was monitored in-vivo by the respective bioluminescent reporters. Around day 4 and 6, CD4+ TCs migrated from tumor-draining lymph nodes into the tumor environment and persisted until rejection. Interestingly, activation of CD4+ TCs was only transient (between days 4 and 7) in all mice, independent of therapy outcome (in Figure B shown for refractory tumor). Whereas loss of activation signal during remission was correlated with tumor clearance and decline of effector function, in refractory tumors it suggests a rapid inactivation of infiltrating TCs by the tumor microenvironment. Our data indicate that the failure of tumor rejection is not caused by impaired peripheral expansion or tumor homing but rather by inhibition of TC effector function. Responsible mechanisms and counter-acting therapeutic interventions are the focus of ongoing studies.
In summary, the BLITC reporter system facilitates analysis of therapeutic parameters for ATT in a well-established solid tumor model. Using BLITC mice for transduction with TCR or CAR expression cassettes could allow rapid monitoring of on-target as well as undesired off-target effects in virtually any tumor setting. Future experiments will focus on the beneficial effects of combination treatments on the activation of adoptively transferred TCs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal