Abstract
Chimeric antigen receptor T cells (CART) hold great promise for the treatment of B cell neoplasms as multiple clinical studies show high complete response rates with anti-CD19 CART. However, widespread clinical use of this novel immunotherapy is limited by cytokine-release syndrome (CRS). CRS is characterized by high fevers and a systemic inflammatory response that is at times fatal. CRS is associated with elevation of multiple inflammatory cytokines (IFNγ, TNFα, IL-6 and others) and may be treated with the anti-IL6 receptor antibody tocilizumab and/or steroids. Importantly, approaches to prevent CRS are currently lacking. The Bruton's tyrosine kinase inhibitor ibrutinib has been approved for relapsing chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL) and is now extensively used in B cell neoplasms. Our group has recently demonstrated that the rational combination of ibrutinib with CART19 leads to enhanced anti-tumor responses in preclinical model of MCL, CLL and B-cell acute lymphoblastic leukemia (ALL). In addition ibrutinib has been shown to modulate T cell cytokine production. We therefore hypothesized that ibrutinib would reduce CART19-mediated CRS without impairing the anti-tumor efficacy of these cells.
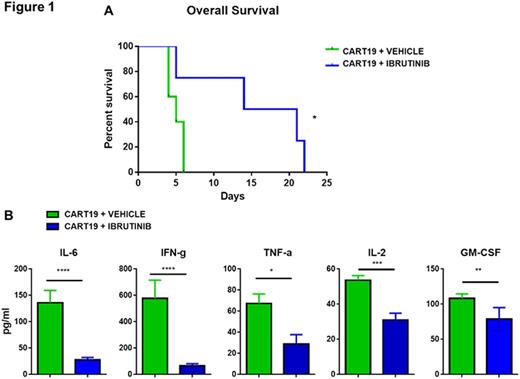
We developed a novel xenogeneic model of CRS by intravenously injecting NOD/SCID/gamma-chain deficient (NSG) mice with primary human MCL cells. Since CRS has been associated with high tumor burden we allowed the tumor to grow for up to 50-60 days, at which time the mice developed palpable splenomegaly. We then injected i.v. 1x106 human CART19 cells or medium (PBS). By day 2 after the infusion, mice that received CART19, but not mice receiving PBS alone, began to display clinical signs of distress (reduced mobility, hyperventilation, generalized weakness, emaciation, hunched bodies, withdrawal and poor motor response) and experienced early death, as compared to controls (p<0.05), clinically resembling CRS. At day 4 after CART19 infusion, we collected blood and measured serum concentration of 30 cytokines by Luminex assay. Importantly, CART19-treated mice, but not control mice, showed significantly elevated serum concentrations of several human cytokines, including IL-6, IFNγ, TNFα, IL-2 and GM-CSF (p<0.05). Having established a clinically relevant model of CRS in the context of B cell neoplasms treated with CART19, we sought to understand if the addition of ibrutinib to CART19 would reduce this early toxicity. We treated high-tumor burden mice with either CART19 plus ibrutinib or CART19 plus vehicle. Of note, mice treated with the CART19/ibrutinib combination experienced prolonged overall survival (OS) as compared to mice receiving CART19 alone (median OS 17.5 days vs. 5 days, p<0.05) (Figure 1 A) Importantly, serum cytokines evaluated on day 4 were significantly reduced in ibrutinib-treated mice, including IL-6, IFNγ, TNFα, IL-2 and GM-CSF (Figure 1 B). In vitro studies revealed that ibrutinib reduced cytokine production by CART cells as well as by MCL cells, leading us to postulate that both CRS and its successful prevention involve cross-talk between immune cells and cancer cells. Studies to dissect the pathogenic pathway are in progress.
In summary, CRS is a major obstacle to the widespread applicability of CART therapy. We here report a novel, clinically relevant xenograft model of CRS developing in B cell neoplasms as a result of CART19 therapy. Furthermore, we show that ibrutinib reduces CART19-mediated CRS and prolongs survival by inhibiting the production of inflammatory cytokines from both CART and tumor cells. Having previously shown that ibrutinib does not impair T cell expansion in vivo and indeed enhances the anti-tumor effect, we suggest that the CART19-ibrutinib combination could be a novel strategy to prevent CRS in B-cell lymphomas as well as in B-cell acute lymphoblastic leukemia. Our institution recently opened clinical trial (NCT02640209) where CART19 (CTL019) is added to ibrutinib in CLL patients who have not achieved a complete response after 6 months.
Ruella:Novartis: Patents & Royalties. Kenderian:Novartis: Patents & Royalties, Research Funding. Melenhorst:Novartis: Patents & Royalties, Research Funding. Wasik:Gilead Sciences: Equity Ownership; Seattle Genetics: Honoraria; Novartis: Research Funding; University of Pennsylvania: Patents & Royalties: NPM-ALK as an omncogene; University of Pennsylvania: Patents & Royalties: CAR T-cells; Gilead Sciences: Research Funding; Pharmacyclics: Research Funding. Lacey:Novartis: Research Funding. June:Novartis: Honoraria, Patents & Royalties, Research Funding; Celldex: Consultancy, Equity Ownership; Tmunity Therapeutics: Equity Ownership; Immune Design: Consultancy, Equity Ownership; Pfizer: Honoraria; Novartis: Honoraria, Patents & Royalties, Research Funding; Johnson & Johnson: Honoraria. Gill:Novartis: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal