Abstract
INTRODUCTION: In utero hematopoietic cell transplantation (IUHCT) is a non-myeloablative, non-immunosuppressive transplant approach that takes advantage of the immunologic immaturity of the fetus to engraft donor cells across immune barriers and induce donor specific immune tolerance (DST). Though it has the potential to treat many congenital hematologic disorders, IUHCT is limited by levels of engraftment below those anticipated to be therapeutic for most target diseases, including sickle cell anemia. Thus, a likely initial clinical application of IUHCT is to induce DST to allow for non-myeloablative, non-immunosuppressive postnatal "booster" transplants to increase engraftment. We have previously validated this approach in murine sickle cell, beta-thalassemia, and non-diseased models using low dose total body irradiation and busulfan prior to postnatal bone marrow transplantation (BMT). Although encouraging, less toxic approaches that specifically target the hematopoietic system would make IUHCT + postnatal BMT more applicable. CD45 is a membrane glycoprotein expressed by all leukocytes and hematopoietic stem cells. Recent studies have demonstrated enhanced engraftment in a congenic postnatal transplant mouse model following pre-transplant conditioning with an anti-CD45-saporin (SAP) immunotoxin (Palchaudhuri et al., Nat Biotech 2016; 34:738-45). We hypothesized that mixed allogeneic chimerism achieved by IUHCT can be enhanced after birth by conditioning with an anti-CD45-SAP immunotoxin followed by a same-donor allogeneic BMT.
METHODS: 5x106 BM mononuclear cells from C57Bl/6 mice (H2Kb, CD45.2+) were injected intravenously into gestational day 14 (E14) Balb/c fetuses (H2Kd, CD45.2+). Donor cell peripheral blood (PB) chimerism was confirmed at 4 weeks of age, at which time chimeric mice were injected via tail vein with PBS (control) or CD45.2-SAP. Six days following treatment, chimeric animals were intravenously injected with 30x106 B6Pep3b (H2Kb, CD45.1+) BM cells. Additional control groups consisted of recipients of IUHCT alone without a postnatal BMT and naïve 5 week-old Balb/c mice which did not undergo IUHCT and only received CD45.2-SAP + postnatal BMT. Following postnatal BMT, PB was assessed bi-weekly by flow cytometry for total donor chimerism and the contribution of prenatal and postnatal donor cells to engraftment. Mice were weighed before each bleeding and evaluated for signs of GVHD. Statistical comparison was performed using the Student's t-test for 2 samples at each time point assuming unequal variances.
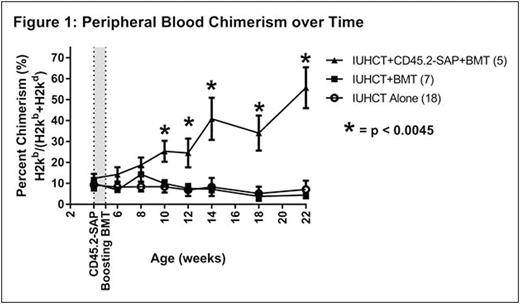
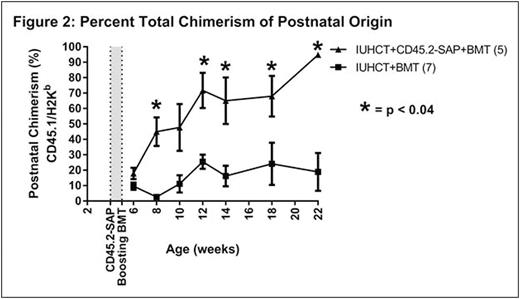
RESULTS: There was no significant difference in initial donor cell chimerism levels at 4 weeks of age between treatment (IUHCT+CD45.2-SAP+BMT, 12.4±2.2%, n=5) and control (IUHCT alone, 9.3±2.7%, n=18; IUHCT+BMT, 10.0±2.6%, n=7) groups prior to therapy. In contrast to naïve non-chimeric Balb/c mice undergoing a postnatal BMT (n=10) which demonstrated no donor cell engraftment, recipients of IUHCT and a postnatal BMT (both PBS and CD45.2-SAP treated groups) demonstrated postnatal donor cell engraftment, supporting DST achieved by IUHCT. While postnatal BMT after IUHCT failed to demonstrate a significant enhancement of engraftment compared to IUHCT alone (4.4±1.3% vs. 7.1±4.2% donor cell engraftment at 22 weeks, p = 0.7), low-level allogeneic chimerism following IUHCT was significantly enhanced to high-level chimerism following conditioning with CD45.2-SAP+BMT (55.7±9.8% vs. 7.1±4.2% donor cell engraftment at 22 weeks, p=0.0001) (Fig. 1). The majority of donor cell chimerism resulted from the postnatal donor population in recipients of CD45.2-SAP, supporting a mechanism of enhanced engraftment via a competitive advantage following recipient CD45.2-SAP treatment (Fig. 2). Chimerism was stable and multilineage at 22 weeks indicating enhanced stem cell/early progenitor engraftment. All mice demonstrated appropriate weight gain without GVHD.
CONCLUSIONS: Prenatal tolerance induction achieved by IUHCT followed by postnatal CD45-SAP conditioning and same-donor BMT results in enhanced stable multilineage allogeneic engraftment at therapeutic levels for many target diseases. This work supports a potentially less toxic approach for the strategy of prenatal tolerance induction to facilitate non-myeloablative, non-immunosuppressive postnatal BMT and has the potential to significantly expand the applicability of IUHCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal