Abstract
Background
Depth of response has been associated in multiple studies with improved PFS and overall survival. Thus, clinicians and patients (pts) are often encouraged by a rapid decrease of M-protein when treatment is initiated. However, little is known about the association of response kinetics with outcomes. Some evidence suggests that early responders may have compromised long-term outcomes compared with slow responders (Boccadoro et al, J Clin Oncol 1998; Lahuerta et al, J Clin Oncol2008). However, these studies were based in the era prior to the availability of novel MM agents. We therefore analyzed long-term outcomes by time to best response and depth of response in TOURMALINE-MM1, a randomized, double-blind, placebo-controlled phase 3 study (NCT01564537) of ixazomib plus lenalidomide-dexamethasone (IRd) versus placebo-Rd in pts with RRMM (Moreau et al, N Engl J Med 2016). The TOURMALINE-MM1 study demonstrated superior PFS with IRd vs placebo-Rd, with limited additional toxicity, and led to the approval by the US FDA of ixazomib, in combination with Rd, for the treatment of pts with MM who have received at least 1 prior therapy.
Methods
722 pts with RRMM after 1-3 prior lines of therapy who were not refractory to prior lenalidomide or proteasome inhibitor-based therapy were randomized 1:1 to receive oral ixazomib 4.0 mg (n=360) or placebo (n=362) on days 1, 8, and 15 of 28-day cycles, plus lenalidomide 25 mg and dexamethasone 40 mg, until progressive disease (PD) or unacceptable toxicity. The primary endpoint was PFS by independent review committee (IRC) assessment based on IMWG uniform response criteria. Response was assessed every cycle based on central laboratory results and by IRC evaluation; treatment was continued until central confirmation of investigator assessment of PD. The study met its primary endpoint at the first pre-specified analysis; a subsequent analysis for survival was performed after a median follow-up of ~23 months, including a non-inferential analysis of PFS. Data reported here are from the 23-month analysis. PFS (measured from study start to progression or death) was analyzed post-hoc in subgroups defined by quality of response and subgroups defined by time needed to achieve the confirmed best response ("time to best response"). To avoid possible guarantee-time bias, duration of best confirmed response (measured from the time of best achieved response until progression or death) was also analyzed.
Results
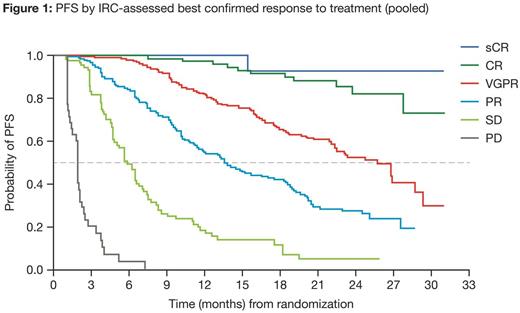
At data cut-off, 676 pts pooled across the IRd and placebo-Rd arms had an IRC-assessed best confirmed response, including 2% stringent CR (sCR), 11% CR, 38% VGPR, 30% PR, 13% stable disease (SD), and 6% PD. Figure 1 shows PFS in pt subgroups defined by quality of response (sCR, CR, VGPR, PR, SD, and PD).
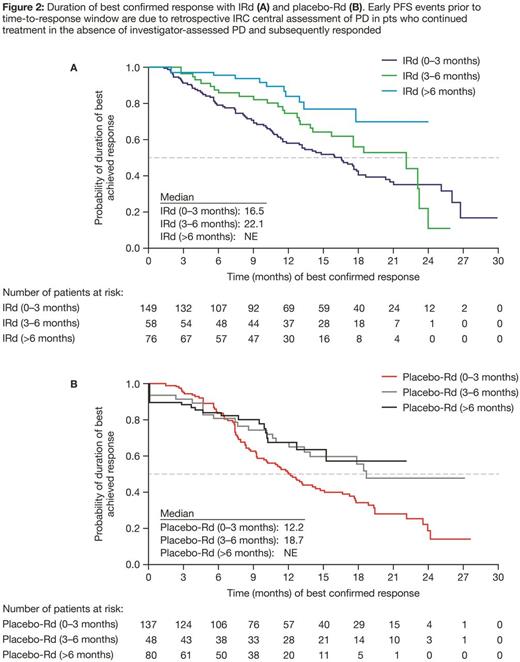
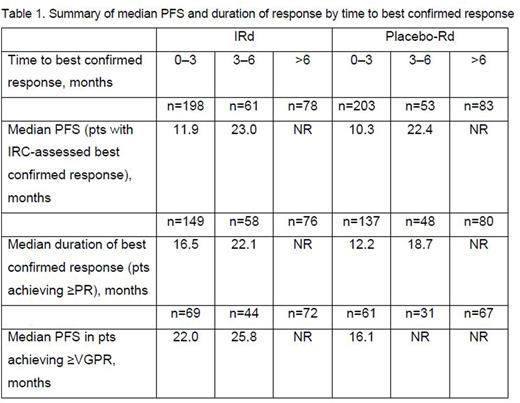
Median time to best response with IRd and placebo-Rd was 2.3 and 2.6 months, respectively; time to best confirmed response was 0-3, 3-6, or >6 months in 198 (59%), 61 (18%), and 78 (23%) pts, respectively, in the IRd arm, and 203 (60%), 53 (16%), and 83 (24%) pts in the placebo-Rd arm. In both the IRd and placebo-Rd arms, median PFS was prolonged among patients with the longer time to best response (Table 1). To address the possibility of guarantee-time bias, the duration of best confirmed response was also analyzed among pts achieving ≥PR, and confirmed a similar trend across both treatment arms (Table 1, Figure 2). Lastly, to address the possibility that pts who only achieved PR could enrich the subset of early responders and thus negatively influence the PFS in this subset (based on the association between depth of response and PFS), a sensitivity analysis was performed among pts achieving ≥VGPR by time needed to achieve VGPR. The median PFS for these patients (Table 1) also supports the trend for longer PFS in patients achieving VGPR later compared to those achieving VGPR early.
Among all pts, rates of grade ≥3 adverse events (AEs) were 73%, 80%, and 72% with IRd, and 70%, 58%, and 69% with placebo-Rd, and rates of serious AEs were 48%, 48%, and 40% with IRd, and 48%, 49%, and 46% with placebo-Rd, in the 0-3, 3-6, and >6 months groups, respectively
Conclusions
These data suggest that longer time to best confirmed response over prolonged duration of therapy, as well as deeper responses, are associated with improved outcomes with both IRd and placebo-Rd in pts with RRMM. Notably, the longer duration of therapy needed to achieve best response did not appear associated with additional toxicity burden.
Garderet:Takeda: Consultancy; Amgen: Consultancy; Bristol-Myers Squibb: Consultancy; Novartis: Consultancy. Stoppa:Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Honoraria; Takeda: Honoraria. Hari:Merck: Research Funding; BMS: Honoraria. Cavo:Bristol-Myers Squibb: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Millennium: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria. Ludwig:Amgen: Research Funding, Speakers Bureau; Janssen: Speakers Bureau; Takeda: Research Funding, Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau. Mateos:Takeda: Honoraria; Amgen: Honoraria; Celgene: Honoraria; Janssen: Honoraria. Luptakova:Takeda Oncology: Employment. Lin:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. van de Velde:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Berg:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Moreau:Novartis: Honoraria; Celgene: Honoraria; Janssen: Honoraria, Speakers Bureau; Takeda: Honoraria; Amgen: Honoraria; Bristol-Myers Squibb: Honoraria. Richardson:Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal