Abstract
Introduction: The Immature Platelet Fraction (IPF) is a generally available hematological parameter, identifying a portion of "young" and hemostatically more active platelets, typically increasing in presence of thrombocytopenia. A high IPF has been associated with JAK2 mutation and increased risk of thrombosis in patients with polycythemia vera (PV) and essential thrombocytemia (ET). However its significance has never been explored in patient with myelofibrosis (MF).
Methods: IPF was measured using the Sysmex XN-Series analyzer; an IPF greater than 6.2% was defined high, in accordance with previous studies. Categorical and continuous variables were compared using the χ2 or Fisher exact tests, and the Mann-Whitney test, as appropriate. Logistic regression was used for multivariable analysis of categorical variable. Survival was calculated using the method of Kaplan and Meier, and univariable comparisons were made using the log-rank test. All p-values < 0.05 were considered significant.
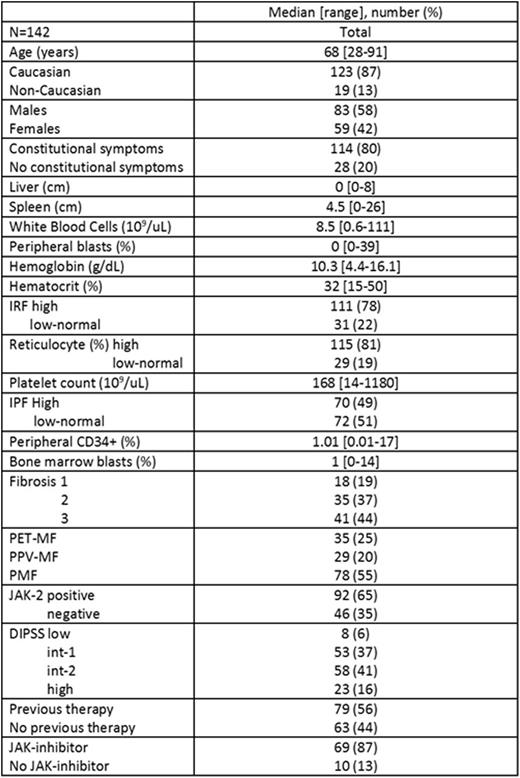
Results. One hundred and forty-two sequential unselected patients with MF were included in the study; baseline characteristics at time of IPF measurement are shown in the Table.
Seventy (49%) patients had a high IPF. Factors associated with high IPF on univariate analysis were elevated white blood count (p=0.04), elevated peripheral blasts (p=0.04), low platelet count (<0.001), secondary MF (p=0.004), JAK2 mutation (p=0.002), and previous therapy (p=0.007). On multivariable analysis, the factors which remained associated with high IPF were elevated peripheral blasts (odd ratio [OR] 3.3, 95% confidence interval [CI] 1.4-8.1; p=0.009), low platelet count (OR 3.7, 95% CI 1.6-8.8; p=0.003), JAK2 mutation (OR 4.3, 95% CI 1.7-10.8; p=0.002) and previous therapy (OR 2.6, 95% CI 1.2-5.8; p=0.02). After a median follow-up of 22 months (range, 1-26) from the date of IPF measurement, no thrombotic events were reported, 11 patients developed secondary acute myeloid leukemia, and 25 patients died. Median overall survival has not been reached. IPF was not significantly associated with any of these outcomes.
Discussion. Similarly to PV and ET, also in patient with MF high IPF associates with JAK-2 mutation, suggesting an interaction between the JAK-STAT pathway and platelet activation in these patients; in addition, high IPF associates with markers of advanced disease, such as elevated peripheral blasts, low platelets and previous exposure to therapy. Longer follow-up is needed to assess the impact of IPF on the risk of thrombosis and transformation, and survival.
Verstovsek:Geron: Research Funding; Pfizer: Research Funding; Genentech: Research Funding; Bristol-Myers Squibb: Research Funding; Promedior: Research Funding; CTI BioPharma Corp: Research Funding; NS Pharma: Research Funding; Lilly Oncology: Research Funding; AstraZeneca: Research Funding; Roche: Research Funding; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Galena BioPharma: Research Funding; Seattle Genetics: Research Funding; Gilead: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal