Abstract
Background:Patients with polycythemia vera (PV) often present with a broad range of clinical characteristics that may contribute to increased risks of cardiovascular (CV) morbidity and mortality, including thrombotic events (TE). Limited contemporary real-world data have been reported about the clinical burden of PV and treatment patterns in the United States. The ongoing REVEAL study collects data on disease burden, clinical management, patient-reported outcomes, and healthcare resource utilization for patients with PV in the United States. This analysis reports clinical characteristics, including underlying CV risk factors, for patients enrolled in the REVEAL study as of April 28, 2016.
Methods: REVEAL is a multicenter, nonrandomized, prospective, observational study enrolling patients ≥18 years of age with a PV diagnosis who are actively managed in an academic or community setting. For this analysis, data regarding PV disease and diagnosis, clinical characteristics, and treatment patterns were collected at enrollment during usual-care visits and were based on physician assessment, electronic medical records, and local laboratory values. Ten-year CV risk factors selected for this analysis were adapted from the Framingham Heart Study for CV diseases.
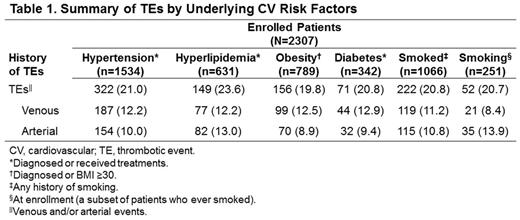
Results: At data cutoff, 2307 patients were available for this analysis. Mean (SD) age was 66.3 (12.2) years, 54.4% were male, 89.9% were white, 62.7% had at least some college education, and 51.1% were retired. Approximately 6% of patients had a family history of PV, primarily in parents (35.1%) and siblings (33.8%). A history of second malignancies was reported for 344 patients (14.9%). The majority of patients (84.6%) were diagnosed with PV based on an abnormal blood test alone or in combination with a bone marrow test. Among patients who were diagnosed with a mutational test (n=1078), 95.2% were diagnosed via an abnormal JAK2V617F test result. Abnormal hemoglobin (57.3%), hematocrit (55.4%), or both (47.5%) were among the most common blood values assessed for PV diagnosis. At diagnosis, 58.5% of patients were classified with high-risk PV (age ≥60 years or history of a TE); this percentage increased to 77.3% at REVEAL enrollment. The average (SD) disease duration from diagnosis to enrollment was 5.8 (6.1) years. At enrollment, 91.5% of patients were under active management for PV (phlebotomy ± aspirin, 34.0%; hydroxyurea ± aspirin, 27.0%; and phlebotomy + hydroxyurea ± aspirin, 23.2%). Underlying CV risk factors that were either diagnosed or treated in 86.0% of enrolled patients included hypertension (66.5%), history of smoking (46.2%), current smoking at enrollment (10.9%), obesity (34.2%), hyperlipidemia (27.4%), and diabetes (14.8%). At enrollment, 431 (18.7%) patients reported having ≥1 TE, including 181 patients who had a TE between PV diagnosis and enrollment. Venous and arterial TEs were reported in 11.1% and 8.6% of patients, respectively. Most commonly reported venous TEs were deep vein thrombosis (5.9%) and pulmonary embolism (2.5%); most common arterial TEs were cerebrovascular arterial thrombosis including transient ischemic attack (5.1%) and acute myocardial infarction (1.7%). Increased rates of TEs were observed among patients with hyperlipidemia (23.6%) and hypertension (21.0%; Table 1), compared with patients who did not have any risk factors (10.5%).
Conclusion: A large proportion of patients in the REVEAL study had 1 or more underlying CV risks, including age, hypertension, smoking, obesity, hyperlipidemia, and diabetes, which may contribute to the risk of thrombosis. Longitudinal data from REVEAL will provide a better understanding of how these factors affect CV outcomes over time.
Stein:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding. Naim:Incyte Corporation: Employment, Equity Ownership. Grunwald:Janssen: Research Funding; Forma Therapeutics: Research Funding; Medtronic: Equity Ownership; Alexion: Membership on an entity's Board of Directors or advisory committees; Ariad: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Oh:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Research Funding; CTI: Research Funding. Paranagama:Incyte Corporation: Employment, Equity Ownership. Cordaro:Incyte Corporation: Employment, Equity Ownership. Sun:Incyte Corporation: Employment, Equity Ownership. Parasuraman:Incyte Corporation: Employment, Equity Ownership. Boccia:Celgene: Consultancy, Honoraria, Speakers Bureau; Amgen/Onyx: Consultancy, Honoraria, Speakers Bureau; Gilead: Speakers Bureau; Genentech: Consultancy, Honoraria, Speakers Bureau; Eisai: Consultancy, Honoraria, Speakers Bureau. Mesa:Ariad: Consultancy; CTI: Research Funding; Gilead: Research Funding; Galena: Consultancy; Novartis: Consultancy; Promedior: Research Funding; Celgene: Research Funding; Incyte Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal