Abstract
Background: Mercaptopurine (MP) and asparaginase (ASP) are critical components in the treatment of acute lymphoblastic leukemia (ALL). Dose-limiting toxicities of the two drugs are common, resulting in therapy interruption, which has been associated with inferior treatment outcome in some studies. However, the interaction between these drugs has not been clearly identified. Merryman et al (Pediatr Blood Cancer 2012) reported that in DFCI ALL 05-01, patients had lower blood counts and more dosage reductions of MP during consolidation therapy (with concomitant ASP treatment) than during continuation therapy (identical treatment without concomitant ASP). Among groups of homogeneously treated patients with ALL, variability in ASP exposure due to inactivating antibodies can affect ASP pharmacodynamics: we have reported that ASP antibodies were associated with lower plasma ASP activity and higher dexamethasone (DEX) clearance, leading to a lower risk of osteonecrosis and a higher risk of CNS relapse (Liu, Leukemia 2012; Kawedia, Blood 2012). Here we studied the possible effect of ASP antibodies on MP tolerability in St. Jude Children's Research Hospital Total XV, a clinical trial featured intensive ASP treatment.
Methods: A total of 390 children with ALL treated on St. Jude Total XV protocol were evaluable. TPMT genotype was used to guide starting doses of MP. During maintenance treatment, planned MP doses were higher on the low-risk arm (LR; n = 202) than on the standard/high-risk arm (SHR; n = 188). MP dose intensity was estimated as (prescribed dose)/(protocol dose) for weeks 1-146 (boys) or 1-120 (girls) for patients on the LR and SHR arms of maintenance therapy.
Native E.coli-ASP (Elspar) was administered intramuscularly at 10000 U/m2 thrice weekly for 6 or 9 doses during remission induction. During maintenance therapy, patients on the LR arm received ASP only during reinductions I (weeks 7-9) and II (weeks 17-19), whereas those on the SHR arm received 19 weekly doses at 25000 U/m2 during weeks 1-19. Patients were tested for serum anti-Elspar antibodies at days 5, 19, 34 of remission induction, day 1 of reinduction I and day 1 of reinduction II, and were grouped based on whether they were ever positive for antibodies at any time during therapy or not. The area under the antibody concentration-time curve (AUC) for the entire period up to week 19 was also estimated in 360 patients.
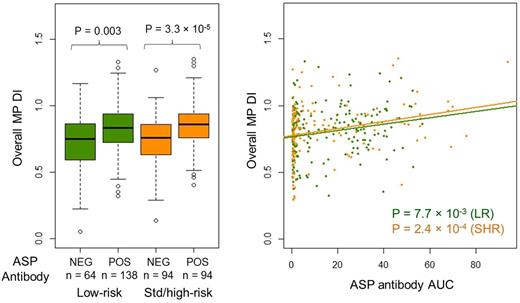
Result: Overall MP dose intensity was higher in those with vs without ASP antibodies in patients on the LR (median 83 vs 75%, P = 0.003) and SHR arms (median 86 vs 76%, P = 3.3 × 10-5; Figure 1A), and MP dose intensity was correlated with ASP antibody AUC in patients on both treatment arms (LR, P = 7.7 × 10-3 and SHR, P = 2.4 × 10-4; Figure 1B). In a multivariate model including age, sex, risk arm, ancestry, TPMT status, NUDT15 genotype and ASP antibody status, TPMT genotype was the strongest determinant of MP dose intensity (-17% in heterozygotes, P = 1.9 × 10-8), followed by ASP antibody positivity (+8.9% dose intensity in those with antibodies, P = 5.8 × 10-6). The model also confirmed previously identified associations of higher MP dose intensity with higher African ancestry (Bhatia et al. Blood 2014) (P = 1.8 × 10-4) and lower Asian ancestry (P = 0.05) (Yang et al. J Clin Oncol 2015).
Conclusion: Interindividual differences in ASP systemic exposure, as reflected by ASP antibodies, had a strong impact on MP tolerance, especially in patients on the SHR arm who received intensive ASP therapy. We have previously shown that patients who are positive for ASP antibodies not only have lower exposure to ASP but also to dexamethasone (Kawedia, Blood 2012; Liu, Leukemia 2012). These data further emphasize the capacity for variation in ASP exposure to impact yet another critical component of ALL therapy.
Asparaginase antibodies associated with higher mercaptopurine tolerance in patients on the low-risk (n = 202) and standard/high-risk (n = 188) arms.
P values were estimated using the (A) Mann-Whitney U test and (B) linear regression model. DI, dose intensity; MP, mercaptopurine; ASP, asparaginase; NEG, anti-asparaginase antibody negative; POS, anti-asparaginase antibody positive.
Asparaginase antibodies associated with higher mercaptopurine tolerance in patients on the low-risk (n = 202) and standard/high-risk (n = 188) arms.
P values were estimated using the (A) Mann-Whitney U test and (B) linear regression model. DI, dose intensity; MP, mercaptopurine; ASP, asparaginase; NEG, anti-asparaginase antibody negative; POS, anti-asparaginase antibody positive.
Evans:Prometheus Labs: Patents & Royalties: Royalties from licensing TPMT genotyping. Relling:Prometheus Labs: Patents & Royalties: Royalties from licensing TPMT genotyping.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal