Abstract
BACKGROUND: Mixed phenotype acute leukemia (MPAL) as defined according to the World Health Organization 2016 guidelines (WHO2016) comprises approximately 2-3% of acute leukemia. In other acute leukemias, minimal residual disease (MRD) has emerged as the predominant predictor of relapse. MRD at end of Induction (EOI) is therefore a key risk-stratifying feature for lymphoid and myeloid disease regimens. To our knowledge, no studies to date have reported on the predictive value of MRD for treatment of MPAL. Although the absence of randomized trials for MPAL precludes a clear determination of optimal regimen, acute lymphoblastic leukemia (ALL) directed-therapy is increasingly considered frontline treatment internationally. The primary objective for this study was to evaluate the association of EOI MRD with event free survival (EFS) from ALL regimens used for treatment of MPAL.
METHODS: Flow cytometry data for all acute leukemias diagnosed 2008-2015 and treated with Children's Oncology Group regimens for ALL were examined by two flow cytometrists blinded to clinical outcomes (MO, SL). Data was analyzed using CellQuestPro and FACSDiva software (BD Biosciences, San Jose, CA). A comprehensive panel of 21 B-, T-, and myeloid markers were evaluated in all cases. A diagnosis of MPAL was strictly established according to the WHO2016 definition. EOI MRD was performed routinely by flow cytometry using a "difference from normal" approach. Cases with insufficient flow cytometry to either (1) establish the diagnosis of MPAL or (2) evaluate the presence and quantity of MRD were excluded. Due to the rarity of the T/myeloid phenotype in the dataset (n=1), the analysis was restricted to B/Myeloid. The primary endpoint was EFS; secondary outcomes explored predictors of EOI MRD positivity and the prevalence of recurrent cytogenetic abnormalities. MRD was categorized as a binomial (negative <0.01%) or ordinal (<0.01%, <0.1%, ≥0.1%) variable in separate models. EFS was calculated by Kaplan-Meier analysis. Step-wise multivariable Cox proportional hazard models included MRD and demographic/disease candidate predictors (age [years], sex, initial white blood cell count [WBC], central nervous system disease, cytogenetic abnormality, isolated myeloperoxidase expression). As some patients were classified as MPAL retroactively, category of treatment regimen (ALL high-risk [36/53] versus standard-risk [17/53]) was "forced" into the final multivariable model. Due to the non-normal distribution of MRD, predictors of MRD positivity were assessed by rank-sum test.
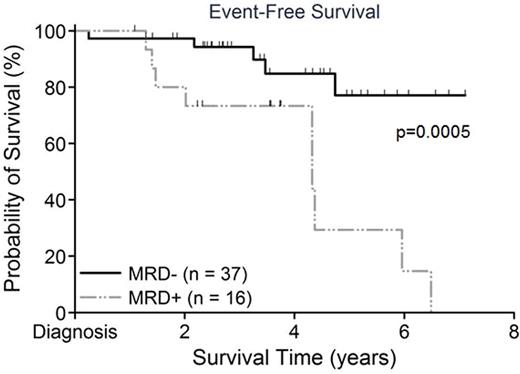
Results: Of 59 cases identified as MPAL, 53 met inclusion criteria for analysis (1 Induction death, 1 insufficient MRD for analysis, 2 received AML therapy, 1 T/myeloid, 1 MPAL lymphoma). At a mean follow-up time of 3.5±1.7 years, EFS for the overall cohort was 88.0±4.6%. However, EFS was significantly greater for those MRD- versus those MRD+ (94.4±3.9% vs 67.5±12.1%, p=0.0005; Figure 1). Multivariable analysis confirmed MRD as a significant predictor of EFS even after accounting for treatment regimen (hazard ratio 5.0, 95%CI 1.7-15.3, p=0.0036). No other candidate predictors remained significant in the final multivariable models. Although different levels of MRD positivity were significantly associated with poorer EFS, visual examination of the dataset showed the 0.01% threshold to be most representative. Recurrent cytogenetic abnormalities were found in 15/53 children (Trisomy 4+10 n=11/53, TELAML n=1/53, iAMP21 n=2/53, BCRABL n=1/53). No MLL rearrangements were identified. The single patient with BCRABL received continuous imatinib during therapy (no event). No differences at diagnosis were present in those EOI MRD+ versus MRD-, although a WBC count ≥50K/uL was borderline significant (p=0.051).
Conclusion: Using a "difference from normal" approach, EOI MRD at a threshold of 0.01% significantly predicts risk for strikingly poorer EFS in children with B/Myeloid MPAL. Children who achieve an EOI MRD-negative remission demonstrate excellent survival with ALL chemotherapy alone. Future investigation is necessary to prospectively validate these findings, examine MRD in T/myeloid and cytogenetic subgroups, and to explore the success of myeloid salvage therapy ±SCT to "rescue" those with a poor response to ALL therapy.
Event free survival by end of Induction MRD status (negative <0.01%)
O'Gorman:Becton, Dickinson and Company: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal