Abstract
Background: E.coli L-asparaginase (L-ASP) is an important component of treatment for childhood acute lymphoblastic leukemia (ALL), but the optimal preparation and dosing remain to be determined. Pegaspargase (SS-PEG) is a pegylated L-ASP formulation commonly used in frontline therapy. Calaspargase pegol (SC-PEG) is a novel formulation that uses the same ASP enzyme and PEG moiety as SS-PEG but a different linker molecule that is more hydrolytically stable, leading to a longer half-life. On Dana-Farber Cancer Institute (DFCI) ALL Consortium protocols, patients (pts) typically receive a single dose SS-PEG during induction, and then 15 doses every 2-weeks (wks) during post-induction in order to maintain therapeutic serum asparaginase activity (SAA), defined as ≥ 0.1 IU/mL, for 30 consecutive wks. We hypothesized that SC-PEG could be administered less frequently than SS-PEG during post-induction therapy with a similar SAA and toxicity profile.
Methods: Between 2012-2015, pts aged 1-21 years with newly diagnosed ALL or lymphoblastic lymphoma (LL) were eligible to enroll on DFCI ALL Consortium Protocol 11-001. Pts were randomized at study entry to receive either SS-PEG (N=120) or SC-PEG (N=119), each given intravenously (IV) at a dose of 2500 IU/m2. Both groups received a single dose during multi-agent remission induction. Post-induction, pts assigned to SS-PEG received 15 doses every 2-wks and those assigned to SC-PEG received 10 doses every 3-wks along with other risk-stratified chemotherapy. Serum samples were obtained 4, 11, 18 and 25 days after the induction dose to determine SAA and prior to each post-induction dose (2 wks after each SS-PEG and 3 wks after each SC-PEG dose) to determine nadir SAA (NSAA) by a validated biochemical assay. Pts were switched to Erwinia asparaginase for Grade 2 or higher allergy or for silent inactivation (defined as 2 consecutive non-detectable NSAA). Asparaginase was permanently discontinued for pancreatitis and held for thrombosis (but re-started once the clot improved). End-induction minimal residual disease (MRD) was assessed in ALL pts by IGH/TCRPCR assay, with low MRD defined as < 0.001.
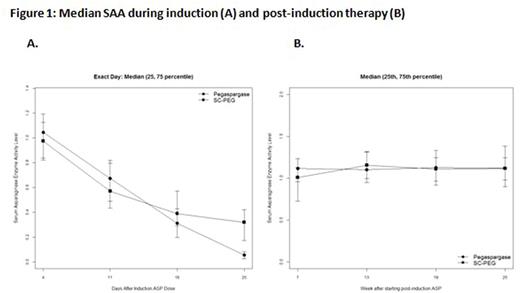
Results: 239 eligible pts were enrolled (230 ALL and 9 LL). There were no significant differences in presenting characteristics between randomized arms. SAA during induction and NSAA during post-induction are displayed in Figure 1. SAA was similar for the two preparations at 4, 11 and 18 days after the induction dose, with SAA ≥ 0.1 IU/mL in ≥ 95% of pts at these time points on both arms. 25 days after the induction dose, SAA was higher with SC-PEG (median 0.298 IU/mL vs 0.056 for SS-PEG), with significantly more pts on SC-PEG arm with SAA ≥ 0.1 IU/mL (88% vs 15%, p<0.0001). Post-induction NSAA was similar between arms, with median NSAA ≥ 1.0 IU/mL (10-times higher than goal NSAA) at 7, 13, 19 and 25 wks after beginning the 30-wk post-induction asparaginase treatment. NSAA was ≥ 0.1 IU/mL in ≥ 98% of pts on both arms at each time point. Two pts on the SC-PEG arm (1.7%) and none on the SS-PEG arm met criteria for silent inactivation. There was no significant difference in rates of ASP-related allergy (p=1.00), pancreatitis (p=1.00), thrombosis (p=0.22) or infections (p=0.86) during induction or post-induction treatment (Table 1). Of 230 evaluable pts, 97% achieved CR, with no difference in proportion of pts with low end-induction MRD by randomized arm (91% SC-PEG vs 90% SS-PEG, p=1.00).
Conclusion: During remission induction, a single dose of SC-PEG (2500 IU/m2) leads to more sustained SAA without excess toxicity or significant difference in the proportion of pts with low end-induction MRD. During post-induction therapy, SC-PEG can be given less frequently (every 3-wks) than SS-PEG (every 2-wks) with similar NSAA and toxicity. The high NSAA observed during post-induction therapy with each preparation suggests that a longer dosing interval and/or reduced dose may be feasible while still maintaining NSAA ≥ 0.1 IU/mL. Longer follow-up is necessary to determine event-free survival by randomized arm.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal