Abstract
Protein disulfide isomerase (PDI), secreted by platelets and endothelial cells upon vascular injury, is required for thrombus formation. However, the precise mechanism by which PDI regulates thrombosis remains elusive. Using PDI variants that form stable mixed disulfide complexes with their substrates, we performed kinetic trapping experiment in platelet rich plasma and identified multiple substrate proteins for PDI, including vitronectin. Importantly, when using variants of endoplasmic reticulum protein 57 (ERp57), a thiol isomerase that has a similar domain structure as PDI and is also important for thrombus formation, the trapping mutants of ERp57 do not interact with vitronectin. This result has demonstrated the substrate specificity of PDI during our kinetic trapping experiment. Further study using polyethylene glycol (PEG)-based gel mobility shift assay combined with mass spectrometry has identified the redox reaction between PDI and vitronectin occurs on two disulfide bonds Cys 137-161 and Cys 274-453 in the hemopexin-like domains of plasma vitronectin.
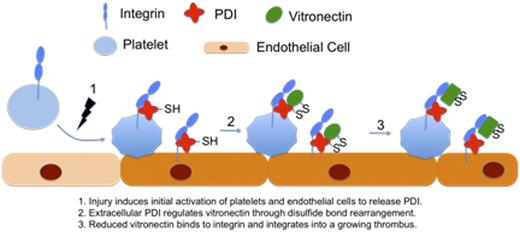
Vitronectin, as a substrate of extracellular PDI, has been shown to be important for thrombus formation. Vitronectin null mice have reduced platelet accumulation and fibrin deposition in the cremaster arterioles following laser injury. Vitronectin null mice also have significantly prolonged large-vessel thrombosis in the carotid artery using the ferric chloride thrombosis model. Using intravital microscopy we showed that vitronectin rapidly accumulates in a growing thrombus following vessel injury. When mice are treated with eptifibatide to eliminate platelet accumulation, we still observe significant amount of vitronectin accumulation on the vessel wall in the absence of platelet thrombus. This observation was further confirmed using confocal intravital microscopy. After 3D reconstruction of a growing thrombus in mouse cremaster arteriole, vitronectin was identified to locate primarily on the CD31 stained vessel wall. These combined studies suggest that plasma-derived vitronectin and not platelet-derived vitronectin is the primary substrate of PDI.
Our study further showed that the indispensable role of vitronectin to a growing thrombus depends on extracellular PDI. Native plasma vitronectin does not bind to αvβ3 or αIIbβ3-integrins on endothelial cells and platelets. On solid phase binding assay, plasma sample pre-treated with wild-type PDI showed significantly increased binding of vitronectin to its ligand αvβ3 or αIIbβ3-integrins. However, this increase was not observed in plasma pre-treated with dead-mutant PDI or ERp57. In addition, using immunofluorescent staining, PDI treated plasma sample also showed significantly increased binding of vitronectin to activated human umbilical vein endothelial cells (HUVECs) and this binding was abrogated by RGD peptides or an αvβ3 blocking antibody. The critical role of extracellular PDI for the regulation of vitronectin in a growing thrombus was further confirmed in our in vivo studies. When mice were treated with quecetin-3-rutinoside or two different inhibitory antibodies that selectively block PDI activity, the accumulation of vitronectin and platelets was significantly reduced. These combined results demonstrate that extracellular PDI regulates vitronectin in a growing thrombus to promote platelet accumulation and fibrin generation.
In summary, our studies have revealed a novel regulatory mechanism during the initiation of thrombus formation. Under normal physiologic conditions in the absence of secreted PDI, thrombus formation is suppressed and maintains a quiescent, patent vasculature. The release of PDI during vascular injury serves as a novel regulatory switch that allows activation of proteins, including vitronectin, which are critical for the following platelet accumulation and fibrin generation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal