Abstract
Background:
Venous thromboembolism (VTE) is a well-known complication in adults receiving asparaginase (ASNase) based intensification chemotherapy for acute lymphoblastic leukemia (ALL). We previously reported a high VTE rate in patients receiving a modified Dana Farber Cancer Institute (DFCI) intensification phase, which included weekly ASNase. We report thrombosis rates during induction and the results using two different dosing schedules of enoxaparin as primary VTE prophylaxis in adults treated with the same protocol.
Methods:
We reviewed pts who received induction chemotherapy, without VTE prophylaxis, using DFCI protocol (containing one dose of ASNase) between 2012-2016. Pts achieving complete remission (CR) subsequently received weekly ASNase-based modified DFCI intensification phase for at least 7 cycles (21 weeks) with VTE prophylaxis, using two dosing schedules in consecutive cohorts: A. Low-dose group received enoxaparin 40 mg subcutaneously (SC) daily for patients weighing < 80 kg and 60 mg daily for those ≥ 80 kg; B. Dose-escalated group received enoxaparin 1 mg/kg SC daily (rounded to the nearest 20 mg).
VTE rates were calculated for pts during induction and intensification phase (low-dose and escalated dose prophylaxis). Results were compared to a similar group of 99 pts previously treated with the same DFCI protocol who did not receive VTE prophylaxis during intensification. Patients not achieving CR, relapsing, undergoing alloSCT after induction, not completing at least 21 weeks of intensification phase or developing VTE before induction were not included.
Results:
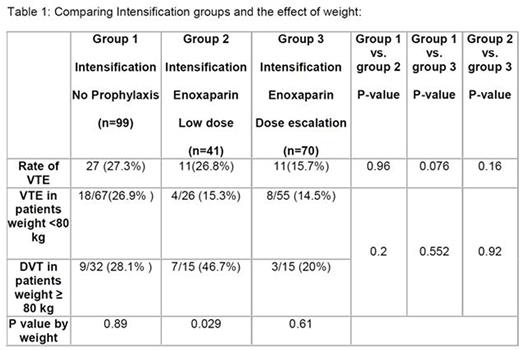
The VTE rate during induction (n-=144) was 2.8%. Of 111 pts who received intensification prophylaxis, the overall VTE rate was 19.8% (p<0.001 compared to induction). Of 41 patients who received low-dose prophylaxis, the VTE rate was 26.8%, while the VTE rate in the dose-escalated prophylaxis group was 15.7%. This compared to a 27.3% rate in the historical non-prophylaxis group (Table 1). There was no significant difference among intensification groups (no prophylaxis, low-dose, and dose-escalated) with respect to median age, gender, weight and number of treatment cycles.
The actual mean dose of enoxaparin in the low-dose prophylaxis group was 0.62 mg/kg, as compared to 0.90 mg/kg in the dose-escalated group. There were no major bleeding complications observed in the prophylaxis groups. The minor bleeding rate in the entire prophylaxis cohort was 4.5% (5/111), and was similar between the low-dose and escalated dose groups. Sites of VTE in the prophylaxis groups included lower extremity (11 cases), sagittal sinus (3), subclavian line related (5), pulmonary embolism (9), and cardiac thrombus (1); some patients had more than one site involved.
Conclusions:
Our data confirmed a high VTE rate during intensification, even with prophylaxis. Dose-escalation of enoxaparin to 1 mg/kg was safe with a trend toward reduction in VTE rates, particularly in patients weighing > 80 kg; however, a larger cohort would be needed to determine if this difference is significant. The use of novel anticoagulants in this setting could be considered.
Schuh:Amgen: Membership on an entity's Board of Directors or advisory committees. Yee:Novartis Canada: Membership on an entity's Board of Directors or advisory committees, Research Funding. Gupta:Novartis: Consultancy, Honoraria, Research Funding; Incyte Corporation: Consultancy, Research Funding. Schimmer:Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal