Abstract
Background
Patients undergoing cord blood transplants (CBT) for hematologic malignancies are known to be at risk for significantly delayed neutrophil and platelet engraftment, resulting in an increased risk of transplant related mortality from infections and bleeding complications. We have previously shown that infusion of CD34+ hematopoietic stem/progenitor cells (HSPC) expanded ex-vivo significantly shortens the time to neutrophil engraftment when compared to conventional CBT. Here, we retrospectively analyzed whether infusion of ex-vivo expanded CD34+ cells with one or two unmanipulated cord blood (CB) units reduces both the time to platelet engraftment and the number of platelet and red blood cell transfusions required within the first 100 days post-transplant for patients undergoing myeloblative CBT.
Methods
Between April 2006 and December 2015, 123 patients on research protocols underwent a CBT with myeloblative conditioning (fludarabine 75 mg/kg, cyclophosphamide 120 mg/kg, and 13.2 Gy TBI) with or without infusion of an ex-vivo expanded cord blood (CB) CD34+ HSPC product. Time to platelet engraftment, units of platelets transfused, and units of red blood cells transfused through 100 days post-transplant were determined for each patient. The time to platelet engraftment was defined as the first day the post-transplant platelet count was greater than 20,000 without a platelet transfusion for 7 consecutive days. A two sample t-test was applied to compare the time to platelet engraftment and the number of platelet and red blood cell transfusions through 100 days post-transplant between two cohorts, conventional and expanded. Cumulative incidence of platelet engraftment was calculated using the Fine and Gray hazard regression model and considered death and relapse as competing risks, controlled for weight, gender, and CBT group.
Results
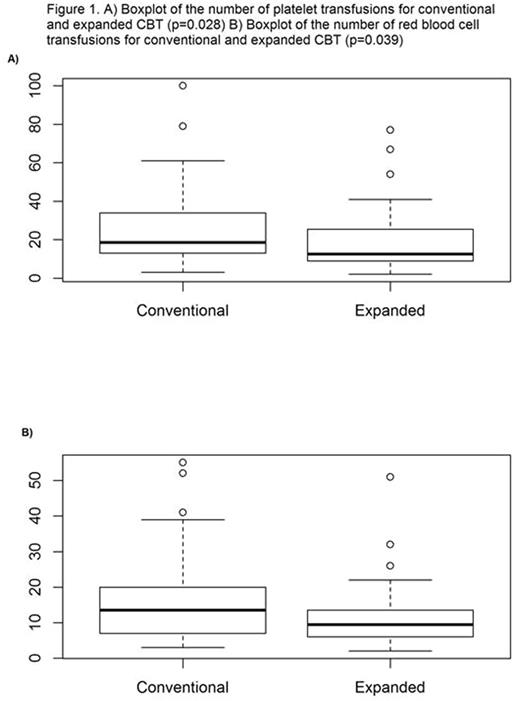
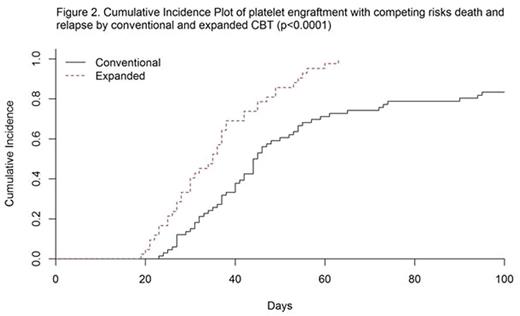
One hundred twenty-three consecutive patients, median age 22 years (range 7 mos - 45 yrs), being treated for AML (n=54), ALL (n=49), MDS/myeloproliferative disorders (n=9), and other malignancies (n=11) were included in the study. No significant differences between the two cohorts were found with respect to weight, age, sex, disease, and diagnosis. Seventy-four (60%) patients received a conventional CBT with 1 (n=18) or 2 (n=56) unmanipulated CB units, while 49 (40%) patients received a CBT with ex-vivo expanded CD34+ HSPC in addition to 1 (n=14) or 2 (n=35) unmanipulated CB units. Median time to platelet engraftment for the conventional vs the expanded cohorts was 43 days and 35 days, respectively (p=0.0009). Median number of platelet transfusions through day 100 for the conventional vs the expanded cohorts was 18.5 and 12.5, respectively (p=0.028) (Figure 1A). Median number of red blood cell transfusions through day 100 for the conventional vs the expanded cohorts was 13.5 and 9.5, respectively (p=0.039) (Figure 1B). From the competing risk hazard regression model, patients in the expanded cohort vs the conventional cohort had significantly higher likelihood of reaching platelet engraftment (HR 2.45 - 95% CI: 1.66 to 3.60 and p-value <0.0001). At day 40, the cumulative incidence of platelet engraftment was 69% for the expanded cohort and 38% for the conventional cohort. At day 60, the cumulative incidence of platelet engraftment was 98% for the expanded cohort and 71% for the conventional cohort (Figure 2).
Conclusion
The infusion of ex-vivo expanded CD34+ HSPC in conjunction with one or two unmanipulated CB units significantly decreases the time to platelet engraftment as well as the number of platelet and red blood cell transfusions required within the first 100 days post-transplant. By reducing the duration of thrombocytopenia, administration of ex-vivo expanded CD34+ HSPC may protect patients from potentially fatal bleeding complications, improve their quality of life, and importantly reduce the cost of post-transplant transfusion requirements.
Delaney:Nohla Therapeutics: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal