Abstract
Introduction: Patients with disease relapse after allogeneic transplantation (alloHSCT) have a poor prognosis. Donor lymphocyte infusion (DLI) is one of the main clinical options to salvage patients in relapse after transplant. Cytokine Induced Killer (CIK) cells are in vitro activated and expanded T lymphocytes which have acquired NK like cytotoxicity as well as CD56 expression. CIK cells have shown Graft versus Leukemia (GvL) activity with little GvHD and therefore may represent an ideal candidate to treat post-transplant relapse. We report the final results of a phase II multicenter pilot study, whose objective was to evaluate the safety and efficacy of sequential administration of donor derived unmanipulated DLI and CIK cells in patients with recurrent hematologic cancers after alloHSCT.
Methods
Seventy-four patients relapsed after alloHSCT, performed using either a matched related (N=42) or unrelated donor (n= 32), were enrolled in the study. This phase II multicenter study was authorized by Istituto Superiore di Sanità, as for Advanced Therapeutic Medicinal Product (ATMP) regulations, and approved by the Agenzia Italiana del Farmaco (AIFA). The trial was registered as (EUDRACT number 2008-003185-26, ClinicalTrial.gov : NCT01186809).
Results
Among the 74 patients (including 16 children and 58 adults) enrolled into this study (median age 45, range 1-67), 20 had a diagnosis of ALL (27%), 41 of AML (55%), 4 of MM (5%), 3 of HD (4%), 2 of NHL (3%) and 4 of MPN (5%). All patients relapsed after matched allogeneic transplants (32 unrelated and 42 sibling), of whom 44 (59%) suffered from a hematological, 4 (5%) from a cytogenetic and 26 (35%) from a molecular relapse. The therapeutic strategy consisted of two infusions of unmanipulated DLI (each of 1 x 106/kg cells) at 3 weeks interval, followed by three infusions of donor derived CIK cells given at 3 weeks interval. The first 12 patients were treated with increasing numbers of CIK cells, in groups of three patients per dose level. Since dose limiting toxicity (DLT) was never observed (acute GVHD of grade III or more), the highest dose planned (5 x 106/kg, 5 x 106/kg and 10 x 106/kg) was then administered to all patients.
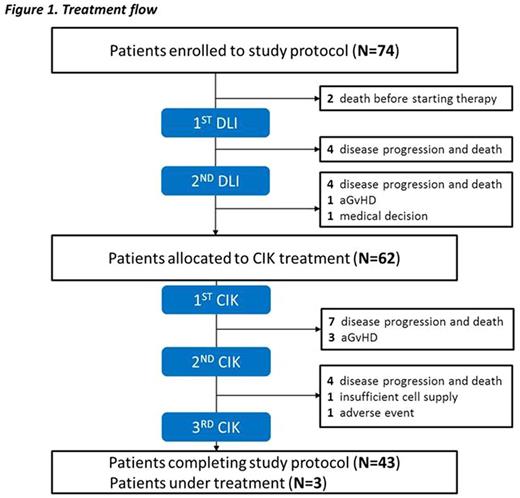
Ten patients died for disease progression, 1 patient developed aGVHD (grade I, skin only) and 1 withdrawn for medical decision before or during the DLI treatment and could not proceed to the planned subsequent CIK administration. Sixty-two patients received at least one infusion of CIK cells, of whom 43 patients (61%) completed the cell therapy program, while 3 patients are still under treatment. The study flow is outlined in Figure 1.
As per protocol, clinical response was determined 100 days after the last CIK administration and the study was analyzed on an intent to treat basis. An early death occurred in 24 (32%) patients (4 during the DLI), no response was observed in 18 (24%) patients, a stable disease in 1 patient (1%), a complete remission in 21 (28%) and a partial remission in 6 (8%), for an overall response rate of 36%. In 4 patients clinical response could not be evaluated (3 patients still in treatment and 1 withdrawn from the protocol). Acute GVHD was observed in a total of 11 patients (15%): grade 1 (n=4), 2 (n=2) and 3-4 (n=5). During follow up, chronic GVHD was observed in 8 patients (11 %) (3 mild, 4 moderate and 1 severe).
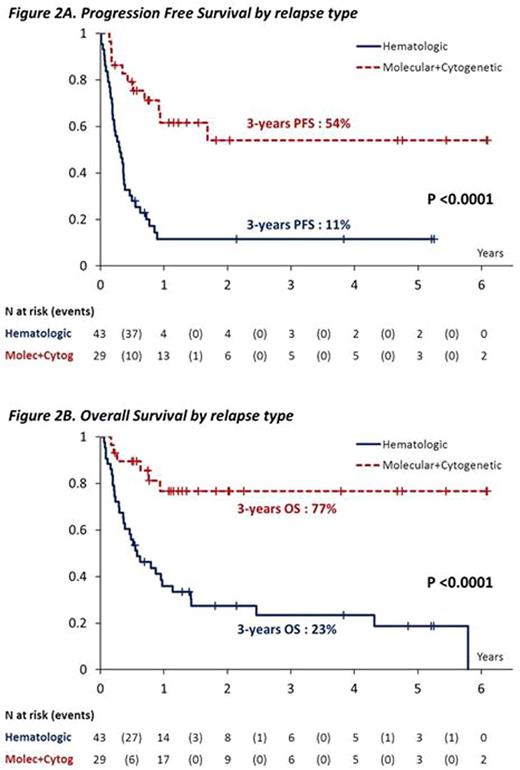
By univariate analysis, progression free survival (PFS) and overall survival (OS) were significantly associated (p<0.0001) with the type of relapse since at 3 years it was 11% and 23% vs. 54% and 77 % for patients enrolled due to a hematologic vs. a molecular/cytogenetic relapses, respectively (Figure 2A-B). By multivariate analysis, the type of relapse remained the only significant predictor of survival (p=0.0019).
Conclusion
Our study shows that administration of CIK cells is feasible in patients with recurrent hematologic cancer after alloHSCT with a relatively low toxicity in terms of GvHD. Particularly in the setting of the molecularly relapsed patients, long-term survival can be achieved. In future studies, we are planning to test CIK cells in preventing post-transplantation relapse in high risk AML. Finally, CIK cells may represent an innovative platform to transduce chimeric antigen receptors in allogeneic T cells with a reduced risk to induce GvHD.
Biondi:Cellgene: Other: Advisory Board; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal