Abstract
Background: Regimens using post-transplant cyclophosphamide (CY) have been developed to provide potent in vivo T cell depletion for patients undergoing human leukocyte antigen (HLA)-haploidentical hematopoietic cell transplantation (HCT). Luznik, O'Donnell and colleagues (BBMT 2008) reported that when this immune suppression strategy is coupled with non-myeloablative conditioning (fludarabine 150 mg/m2, CY 29 mg/kg, 2 Gy total body irradiation) followed by marrow transplantation, it was well-tolerated with low rates of non-relapse mortality (NRM; 15% at 1 year). However, the 2 year overall survival (OS) and event-free survival were low at 36% and 26%, respectively, due to high relapse rates (51% at 1 year). One explanation could be that while post-HCT CY promoted low rates of acute graft-versus-host disease (GVHD), it also eliminated early T and natural killer (NK) cell clones important for disease surveillance. Based on this hypothesis, we developed a next-generation Phase I/II clinical trial incorporating a boost of donor NK cells on day +7 as an attempt to prevent relapse after transplant. In this study, CY 50 mg/kg as a single dose on day +3 was used for T cell depletion.
Methods: Forty patients (pediatric, n=14; adult, n=26) with median age of 45 (range 8-75) years having ALL (n=11), AML (n=9), MDS (n=6), HL (n=6), MM (n=4), NHL (n=3), and CLL (n=1) underwent non-myeloablative transplantation using related, HLA-haploidentical marrow donors on this prospective clinical trial. Patients were high risk due to underlying disease, potential for relapse, and/or risk for transplant-related mortality (TRM). Most patients were heavily pre-treated, with median time from cancer diagnosis to transplant being 2 (0.3 - 12.1) years, including 18 patients having 26 prior HCTs (auto, n=14; allo, n=12). Twenty-five patients (63%) had HCT-CI scores ≥ 3 indicating high risk for TRM.
In order to obtain NK cells, non-mobilized peripheral blood mononuclear cells were collected from donors on day +6 using apheresis and stored overnight. NK cells were isolated on day +7 using the Miltenyi CliniMACS system (CD3 depletion followed by CD56 selection) and were administered as a single, fresh infusion that same day without prior culturing or expansion. The Phase I dose-finding study (n=11) enrolled at 2 NK doses [2.5 or 5 x 106/kg +/- 20%, respectively], with extended enrollment at the 2nd dose level for Phase II (n=29) with 83% of patients meeting NK dose parameters. NK cell products had a median log T cell depletion of 5.4 (4.1-7.1), median NK recovery of 54% (33-68%), and median NK purity of 92% (74-99%). Excellent viability (>95%) was seen in all NK products.
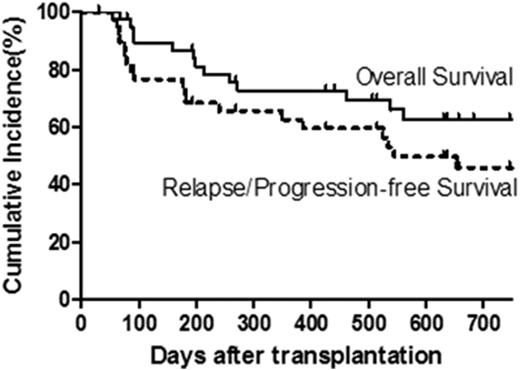
Results: One patient developed chest pain associated with NK cell infusion; otherwise all other patients tolerated their NK cell infusions well without fevers or other adverse reactions. Full donor chimerism (>95% CD3) was seen in 83% of patients at last follow-up, while 18% and 10% experienced graft rejection or graft failure, respectively. Cumulative incidence of grades 2-3 and grade 3 (no grade 4 seen) acute GVHD occurred in 36% and 8% of patients, respectively, at day +100. Of the 39 evaluable patients, 16% developed chronic extensive GVHD at 1 year. Relapse or progression occurred in 31% of patients by 1 year after HCT. With a median follow-up of 1.5 years (range, 0.1 - 4.9 years), 14 patients have died from relapse/progression (n=11) or infection/VOD (n=3), giving a probability of OS, relapse/progression-free survival (PFS), and NRM at 1 year of 73%, 62%, and 8%, respectively, and 2 year OS and relapse/PFS of 63% and 46%, respectively (Fig 1).
Summary: We have demonstrated the safety of infusing donor NK cells early after HCT in a group of heavily-treated patients with high-risk hematological malignancies. In many patients, disease-free survival was possible with the aid of this prophylactic infusion of donor NK cells in combination with allogeneic HCT. These results provide a promising platform to further augment NK cell alloreactivity in the post-HCT setting to prevent relapse and disease progression.
Hari:Merck: Research Funding; BMS: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal