Abstract
Introduction:
MDS include a spectrum of hematopoietic stem cell malignancies characterized by bone marrow failure and dysplastic morphology. LGL is a clonal proliferation of cytotoxic T cells, which manifest as neutropenia, anemia, and thrombocytopenia and is associated with autoimmune disorders. LGL in association with MDS has been previously reported. However, clinicopathological features, prognostic, and predictive factors in those patients diagnosed with both LGL and MDS is not well studied.
Methods:
We identified patients at Moffitt Cancer Center (MCC) diagnosed with MDS who were previously tested for the presence of LGL clonal populations by peripheral blood flow cytometry at time of first visit. An LGL population was defined by the standard flow cytometry immunophenotype and clonality confirmed by T-cell receptor gamma and beta gene rearrangement.. Next Generation sequencing data was available for 151 patients. Recurrent somatic gene mutations were compared between patients with an LGL clone and those without.
Results:
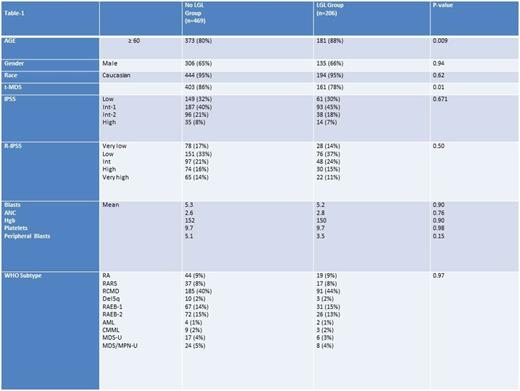
Of the 675 patients with MDS tested for LGL in the database, 206 (30.5%) had an LGL clonal population. The mean LGL absolute cell count in the peripheral blood was 335/µL. Table-1 summarizes the baseline characteristics of the two groups.
There was no difference in response to azacitidine therapy. Among 50 patients with LGL clone who received azacitidine with available data on response, the rate of hematological improvement or better (HI+) was 38%. The (HI+) was 28% among 105 patients evaluable for response without LGL clone. P .14
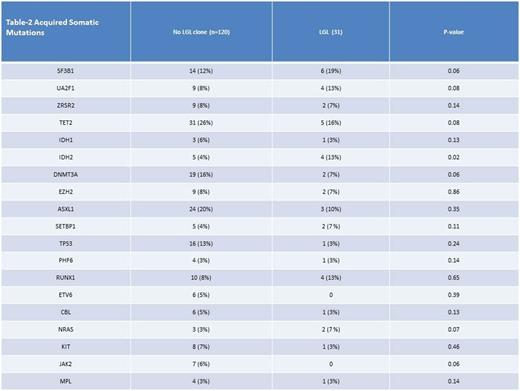
The median overall survival (OS) was for patients with no LGL clone was 65 months (mo) compared to 46 mo (p .024). The median OS for lower risk MDS patients (low/int-1 by International Prognostic Scoring System [IPSS]) was 68 mo versus 97 mo for those with or without LGL proliferation, respectively (P .005). In higher risk MDS, there was no difference in median OS between those with or without LGL expansion, respectively (20 mo versus 16 mo, p .7). The median OS for patients with very low/ low Revised-Internatinal Prognostic Scoring System (R-IPSS) was 96 mo if LGL proliferation was detected compared to 128 mo if it was not, (p value .016). For intermediate R-IPSS the median OS was 65 mo and 41 mo with or without LGL proliferation (p .16). Finally, for high/very high R-IPSS the median OS was 18 and 16 mo with or without LGL proliferation, (p .84) In cox regression analysis the presence of an LGL clone was independently prognostic for OS after adjusting for age and R-IPSS, Hazard ratio 1.3, p = .05. Somatic gene mutation data were available for 151 patients; there was no statistically significant difference in the distribution of any mutation except IDH-2 (Table-2). The most common somatic mutations observed among patients with LGL clone were SF3B1 19%, TET-2 16%, U2AF1 13%, IDH-2 13%, RUNX-1 13%, and ASXL-1 10%. In patients without an LGL clone the most common somatic mutations were TET-2 26%, ASXL-1 20%, DNMT3A 16%, TP53 13%, SF3B1 12%.
Conclusion:
An LGL clone is demonstrable in approximately 30% of patients with MDS in association with advancing age. The presence of LGL proliferation was associated with worse OS in lower risk MDS pts. Although the spectrum of somatic gene mutations were similar, the presence of IDH-2 mutation and absence of DNMT3A or TP53 gene mutationscharacterized LGL+ cases.
Roe:Celgene: Speakers Bureau; Alexion: Speakers Bureau; Seattle Genetics: Speakers Bureau. Sweet:Pfizer: Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Ariad: Consultancy, Speakers Bureau; Incyte Corporation: Research Funding; Karyopharm: Honoraria, Research Funding. Sokol:Seattle Genetics: Consultancy; Spectrum: Consultancy. Komrokji:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Speakers Bureau; Incyte: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal