Abstract
Background:Acute promyelocytic leukemia (APL) is a favorable-risk subgroup of AML characterized by the t(15;17) translocation. The leading cause of early death in APL is uncontrolled bleeding mostly attributed to aberrant expression of tissue factor (F3) and annexin A2 (ANXA2) on leukemic promyelocytes leading to disseminated intravascular coagulation and hyperfibrinolysis, respectively. To prevent or treat such complications, early suspicion of APL and rapid initiation of therapy and supportive measures are critical.
Podoplaninor PDPN is a surface glycoprotein expressed in most cell types (http://www.gtexportal.org), but not in blood cells. CLEC-2, the PDPN receptor, is expressed on normal platelets and was found to be necessary for the separation of blood and lymphatic vessels during embryogenesis. PDPN expression (whether endogenous or ectopic) in cell lines induces platelet aggregation, which can be inhibited by chemical tool compounds or by monoclonal antibodies (Chang et al, Oncotarget, 2015, Kato et al, Biochem. Biophys. Res. Commun., 2006).
Aims and Methods:We analyzed the transcriptome of 30 APL comprised in the Leucegene 430 AML cohort, aiming to identify clinically useful markers and to better understand the hemostasis-related transcriptomic landscape of this subgroup. Patient cohorts and sorted normal hematopoietic cell populations (n=63) were previously reported (Lavallée et al, Nature Genetics, 2015 and Lavallée et al, Blood, 2016). Comparative analysis of gene expression and mutations were performed as previously described.
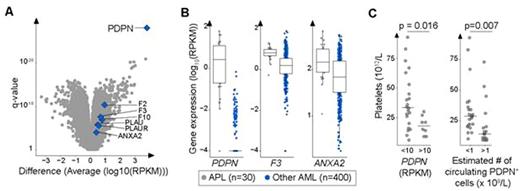
Results:Our analytical pipeline identified several mutated genes in this cohort, most of which are non-specific and previously identified. CEBPE mutations were the only exception and were specific to APL specimens in this cohort (2/30 vs 0/400, p= 0.005). Most interestingly, we identified PDPN as the single most differentially overexpressed gene in APL (median 2.6 vs 0 RPKM, q = 7 x 10-29, Fig A-B). We also found that PDPN is not expressed in whole blood, bone marrow and in any sorted cell subpopulations from these normal tissues, including promyelocytes (median PDPN expression = 0 RPKM, range 0-0.018). This indicates that platelets are never exposed to PDPN in the adult vasculature and reveals that this gene is ectopically expressed in APL promyelocytes. Accordingly, our hypothesis is that aberrant PDPN expression on leukemic promyelocytes contributes to abnormal platelet aggregation in APL patients. We found that high PDPN expression is associated with lower platelet counts at presentation (18 vs 34 x 1012/L, median PDPN expression ≥ 10 vs < 10 RPKM, p = 0.016, Fig C). Furthermore, a strong inverse correlation was observed between the number of estimated circulating PDPN+ promyelocytes and platelet counts (leukocyte count x [% PDPN+ cells] > 1 x 109/L, Fig C, p=0.007). By incorporating anti-PDPN antibody (clone NC-08, Biolegend) in the EuroFlow protocol, we observed that PDPN expression test was 90% sensitive and 100% specific for APL (n= 48 and 50 APL and non-APL primary AML, respectively). Of note, 5 APL cases considered positive expressed low levels of PDPN.
Interestingly, by comparing expression of all coagulation and fibrinolysis genes in APL (n=30) to that of non-APL specimens (n=400), we found that PDPN was the most discriminatory transcript (Fig. A). This result stands in sharp contrast with that found with F3 and ANXA2 which largely overlap in these APL versus non-APL human AML (Fig. A-B). This reinforces the hypothesis that aberrant PDPN expression may be a strong and unappreciated contributor to platelet consumption in APL.
Conclusion:I) CEPBE is recurrently mutated in a small subset of APL patients. II) PDPN is the most specific aberrant transcript in this disease and is a new biomarker for APL. Systematic assessment of podoplanin by flow cytometry in newly diagnosed AML could lead to an earlier detection of unsuspected APL cases. III) PDPN expression by leukemic promyelocytes likely contributes to defective primary hemostasis, representing a new mechanism of APL-related bleeding. This provides a strong rationale for evaluating PDPN-CLEC-2 axis inhibitors in this setting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal