Key Points
The combination of rituximab, lenalidomide, and ibrutinib is associated with a high incidence of rash in follicular lymphoma (all grades 82%).
Efficacy of the triplet appears similar to rituximab-lenalidomide in the same patient population.
Abstract
Chemoimmunotherapy in follicular lymphoma is associated with significant toxicity. Targeted therapies are being investigated as potentially more efficacious and tolerable alternatives for this multiply-relapsing disease. Based on promising activity with rituximab and lenalidomide in previously untreated follicular lymphoma (overall response rate [ORR] 90%-96%) and ibrutinib in relapsed disease (ORR 30%-55%), the Alliance for Clinical Trials in Oncology conducted a phase 1 trial of rituximab, lenalidomide, and ibrutinib. Previously untreated patients with follicular lymphoma received rituximab 375 mg/m2 on days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 4, 6, 8, and 10; lenalidomide as per cohort dose on days 1 to 21 of 28 for 18 cycles; and ibrutinib as per cohort dose daily until progression. Dose escalation used a 3+3 design from a starting dose level (DL) of lenalidomide 15 mg and ibrutinib 420 mg (DL0) to DL2 (lenalidomide 20 mg, ibrutinib 560 mg). Twenty-two patients were enrolled; DL2 was determined to be the recommended phase II dose. Although no protocol-defined dose-limiting toxicities were reported, a high incidence of rash was observed (all grades 82%, grade 3 36%). Eleven patients (50%) required dose reduction, 7 because of rash. The ORR for the entire cohort was 95%, and the 12-month progression-free survival was 80% (95% confidence interval, 57%-92%). Five patients developed new malignancies; 3 had known risk factors before enrollment. Given the increased toxicity and required dose modifications, as well as the apparent lack of additional clinical benefit to the rituximab-lenalidomide doublet, further investigation of the regimen in this setting seems unwarranted. The study was registered with www.ClinicalTrials.gov as #NCT01829568.
Introduction
The standard approach for the treatment of advanced-stage follicular lymphoma, the most common of the indolent non-Hodgkin lymphomas (NHL), has traditionally consisted of chemoimmunotherapy. Although regimens such as R-CHOP (rituximab, cyclophosphamide, vincristine, prednisone) and BR (bendamustine, rituximab) achieve overall response rates (ORR) of >90% in the front-line setting, patients inevitably relapse.1-4 Furthermore, these regimens are associated with acute and long-term toxicity, including but not limited to infection, myelosuppression, and neuropathy. Given the growing number of novel therapies with unique mechanisms of action, a multitargeted biological regimen may improve outcomes with fewer, less severe adverse events in this otherwise incurable disease.
The Alliance for Clinical Trials in Oncology group, which includes the former Cancer and Leukemia Group B (CALGB), previously demonstrated the efficacy of the combination of rituximab and lenalidomide in follicular lymphoma. The CALGB 50401 phase 2 study reported an ORR of 76% (complete response [CR] 39%) and a 2-year time-to-progression of 52% in patients with relapsed disease.5 Preliminary data from the CALGB 50803 study indicated greater activity with the doublet in the front-line setting. Patients received rituximab 375 mg/m2 (cycle 1, days 1, 8, 15, and 22; cycles 4, 6, 8, and 10, day 1) and lenalidomide 20 mg (days 1-21 for twelve 28-day cycles), resulting in an ORR of 96% and a CR rate of 71%.6 An additional 6% achieved a CR by restaging 18FDG PET-CT scans (fluoro-deoxyglucose positron emission tomography combined with computed tomography), but were not included in the reported CR rate becase of lack of confirmatory bone marrow biopsies. The 2-year progression-free survival (PFS) was 89% among the 65 patients enrolled. Fowler et al noted similar response rates in a single-institution study, with a 3-year PFS of 79%.7 These impressive data supported the development of the phase 3 RELEVANCE trial of rituximab-lenalidomide vs rituximab-based chemoimmunotherapy (NCT01476787), which completed accrual in 2015.
The Alliance sought to improve upon the efficacy of rituximab-lenalidomide with the addition of a B-cell receptor antagonist (BCR), ibrutinib. A first-in-class, selective, irreversible inhibitor of Bruton tyrosine kinase (BTK), ibrutinib is approved in chronic lymphocytic leukemia (CLL), mantle cell lymphoma, and Waldenström macroglobulinemia. As a single agent, it has produced ORRs of 30% to 55% in early-phase clinical trials of heavily pretreated patients with relapsed and refractory follicular lymphoma.8-10 Ibrutinib monotherapy is associated with minimal toxicity; the most common adverse events are mild edema, diarrhea, fatigue, and rash, with a small percentage of patients experiencing the more serious side effects of bleeding or atrial fibrillation. Given our mission to develop superior biological alternatives to conventional chemotherapy, the Alliance designed a multicenter phase 1 study of rituximab, lenalidomide, and ibrutinib in previously untreated follicular lymphoma. Utilization of a multitargeted approach against a cell surface marker, the tumor microenvironment, and an intracellular signaling pathway, may improve outcomes in this multiply relapsing disease.
Methods
Eligibility criteria
Patients had previously untreated, histologically confirmed, World Health Organization classification grades 1, 2, or 3a follicular lymphoma, based on central review. Other inclusion criteria included age ≥18 years; Lugano classification bulky stage II (mass ≥7 cm), III, or IV; and Eastern Cooperative Oncology Group performance status of 0, 1, or 2. Patients must have had measurable disease (mass >1 cm) and a clinical indication for treatment at the discretion of their primary oncologist. Required initial laboratory values included an absolute neutrophil count (ANC) ≥1000/µL, platelet count ≥75 000/µL, serum creatinine ≤2 times the institutional upper limit of normal (ULN), estimated creatinine clearance >60 mL/min, total bilirubin ≤1.5 times ULN, and aspartate aminotransferase (AST) and alanine transaminase (ALT) ≤2.5 times upper ULN. Patients with low (0-1), intermediate (2), and high (>3) follicular lymphoma international prognostic index (FLIPI) risk scores were permitted.
Exclusion criteria included prior or concurrent chemotherapy, immunotherapy, radiation therapy, or investigational agents for the treatment of follicular lymphoma; corticosteroids within 2 weeks before study entry unless as maintenance therapy for a nonmalignant condition, or known central nervous system involvement. Prohibited concomitant medications included strong inhibitors or inducers of CYP3A4/5 and vitamin K antagonists. Alternative anticoagulants were permissible. Other exclusion criteria included pregnancy; lactation; active hepatitis B (HBV), hepatitis C, or HIV; active hemolysis; and severe cardiac conditions including uncontrolled arrhythmia, congestive heart failure, myocardial infarction, deep venous or arterial thrombosis within 6 months, and Class III/IV cardiac disease as per New York Hospital Association Functional Classification. Patients with previously diagnosed malignancies were excluded unless treated with curative intent >3 years before screening and felt to be at a low risk of recurrence or had adequately treated nonmelanomatous skin cancer, lentigo maligna melanoma, or cervical carcinoma in situ without current evidence of disease.
Study design
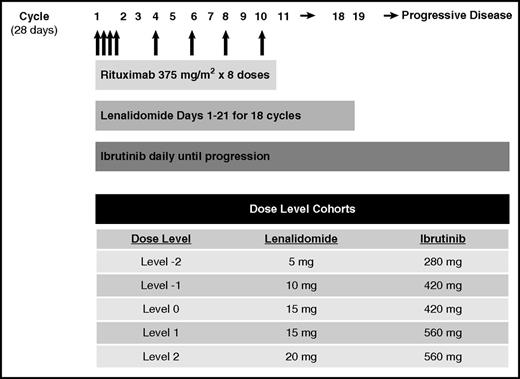
This phase 1 study used a standard 3+3 dose escalation design. Rituximab 375 mg/m2 was administered IV on days 1, 8, 15, and 22 of cycle 1 and on day 1 of cycles 4, 6, 8, and 10. Lenalidomide was administered orally as per cohort dose on days 1 to 21 for eighteen 28-day cycles. Ibrutinib was administered orally as per cohort dose daily until progression or unacceptable toxicity (Figure 1). Patient enrollment began at dose level (DL) 0 (lenalidomide 15 mg and ibrutinib 420 mg) and escalated to DL2 (lenalidomide 20 mg, ibrutinib 560 mg). The maximum dose of lenalidomide was chosen based on efficacy and tolerability in previous front-line combination studies with rituximab in follicular lymphoma.5,6 Ibrutinib 560 mg is the recommended phase 2 dose (RP2D) for investigation in NHL.7,8
Allopurinol 300 mg daily was recommended for tumor lysis prophylaxis with the initiation of therapy, and was discontinued at the discretion of the treating physician. Aspirin was recommended for deep venous thrombosis prophylaxis while receiving lenalidomide. The use of low-molecular-weight heparin was recommended for patients felt to be at a significantly increased risk for venous thromboembolism in addition to aspirin. Granulocyte colony-stimulating factors were restricted to patients who had febrile neutropenia. The use of erythropoietin was not permitted. HBV-suppressive therapy was required for patients with evidence of prior HBV infection. While receiving lenalidomide, female patients of childbearing potential were required to use 2 forms of contraception or practice abstinence in addition to being monitored with pregnancy tests each cycle.
Dose-limiting toxicity (DLT) assessments were performed weekly during cycle 1. Nonhematologic DLTs were defined as any grade 3 or 4 toxicity, any grade Stevens-Johnson syndrome or toxic epidermal necrolysis, grade 3-4 bullous dermatitis, and AST or ALT ≥3 × ULN with total bilirubin ≥2 × ULN. Exceptions included grade 3 or 4 fatigue, anorexia, nausea, fever without neutropenia, or tumor lysis that resolved to baseline after a 2-week treatment delay. Because of the known incidence of rash with lenalidomide, grade 3 rash that resolved to less than grade 2 within 10 days was prospectively excluded as a DLT. Patients may have received supportive care including systemic corticosteroids. A limitation was not set for dosage or duration of corticosteroid. Hematologic DLTs included any grade 4 toxicity (except grade 4 neutropenia lasting <7 days), grade 3 neutropenia with fever ≥38.5°C or infection, and grade 2 or 3 thrombocytopenia complicated by hemorrhage.
Dose modifications and delay were mandated per protocol for specific adverse events. For grade 3 or 4 neutropenia, febrile neutropenia, or grade 3 or 4 thrombocytopenia, treatment with lenalidomide and ibrutinib was interrupted and resumed at one dose level below with the following cycle if toxicity improved to ≤ grade 2. For grade 3 or 4 maculopapular rash, treatment was interrupted and resumed with one dose level reduction when improved to less than or equal to grade 1. Supportive care, including systemic corticosteroids, was permitted without duration or dosage limitations. Study drugs could be held for a maximum of 28 consecutive days for toxicity. Study drugs believed to be responsible for a prolonged toxicity were discontinued in the event of a toxicity lasting >28 days.
Patients underwent a PET-CT scan and bone marrow biopsy at enrollment for initial staging assessment. Response assessments were classified per the International Harmonization Project (IHP) on Lymphoma, which was the standard at the time of study initiation.11 Assessments were determined by the site principal investigator and confirmed by the lead principal investigator. Restaging PET-CT scans were performed at weeks 10, 24, and 52, after which patients underwent CT scans every 4 months for 2 years, followed by every 6 months until progression for 10 years. Bone marrow biopsies were performed in patients who were felt to be in a complete remission and had marrow involvement at baseline.
The study was approved by the ethics committee and institutional review board at all participating cancer centers. Each patient provided written informed consent in accordance with federal and institutional guidelines. The study was registered with ClinicalTrials.gov (NCT01829568). Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was maintained by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. All analyses were based on the study database frozen on April 11, 2016.
Pharmacokinetic blood sampling schedule, measurement of plasma ibrutinib, and PCI-45227 concentrations and pharmacokinetic data analysis
Blood sampling schedule.
Venous blood samples (2 mL into EDTA tubes) were obtained preibrutinib (time 0) and at times up to 24 hours postibrutinib dosing on cycle 1, days 1 and 15. The collected blood samples were processed promptly at 0-4°C and plasma separated, frozen, and stored at −80 °C until analyzed for ibrutinib and PCI-45227 concentrations.
Measurement of plasma ibrutinib and PCI-45227 concentrations.
Plasma concentrations of ibrutinib and PCI-45227 were determined using a validated and specific high-performance liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS) method. The lower limit of quantitation (LLOQ) and the precision (%CV) of the assay in human plasma were 0.5 ng/mL and <11%, respectively.12
Pharmacokinetic data analysis.
Plasma ibrutinib and PCI-45227 concentration-time profiles were initially inspected on a log-linear plot and analyzed using noncompartmental methods (WinNonLn Pro software). The primary pharmacokinetic parameters, observed peak concentration (Cmax), and time to reach the maximum concentration (Tmax), estimated elimination half-life (T1/2), area under the plasma concentration-time curve from time 0 to 24 hours (AUC(0-24)), and accumulation factor R (based on AUC) were defined where adequate data points were available.
Statistical considerations
The primary objective was determining the recommended phase 2 doses (RP2D), also called the maximally tolerated dose (MTD), of lenalidomide and ibrutinib in combination with rituximab in previously untreated follicular lymphoma. Secondary objectives included safety, pharmacokinetics, and preliminary efficacy data. Once the MTD was determined, using the traditional 3+3 cohort method with a potential dose de-escalation, there was a 10-patient expansion cohort at the MTD.
Patient characteristics were summarized using contingency tables for categorical variables; median and range were calculated for continuous variables. Toxicity (attribute and grade) was summarized for the total number of patients, and each dose level as per the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. The observed ORR and CR rates were calculated overall, within dose level, and by FLIPI score. ORR was defined as CR + partial response (PR). Progression-free survival (PFS) was defined as the time from registration until disease progression or death, whichever occurred first. Overall survival (OS) was defined as the time from registration until death. PFS and OS were estimated using the Kaplan-Meier method for the entire study population as well as at the recommended phase 2 dose.13
Results
Patient characteristics
Twenty-two patients were enrolled between June 2013 and August 2014 at 7 Alliance participating cancer centers. Patient characteristics are displayed in Table 1. The median age was 53.5 years (range, 36-81). Sixty-eight percent were male, 27% had WHO classification grade 3a follicular lymphoma, and 77% had stage IV disease. The majority of patients had >4 nodal sites of disease involvement (64%) and bone marrow involvement (77%). Fifty percent of patients had a lymph node >5 cm in one dimension, and 27% had a node ≥7 cm. By FLIPI, 18% were considered low risk, 55% were intermediate risk, and 27% were high risk.
Patient characteristics
| . | Overall . |
|---|---|
| N = 22 (%) . | |
| Median age, y (range) | 53.5 (36-81) |
| Sex | |
| Male | 15 (68%) |
| Female | 7 (32%) |
| Stage | |
| II | 1 (5%) |
| III | 4 (18%) |
| IV | 17 (77%) |
| WHO classification grade | |
| I/II | 16 (73%) |
| IIIa | 6 (27%) |
| ECOG performance score | |
| 0 | 13 (59%) |
| 1 | 9 (41%) |
| FLIPI score | |
| Low risk | 4 (18%) |
| Intermediate risk | 12 (55%) |
| High risk | 6 (27%) |
| Nodal sites | |
| ≤4 sites | 8 (36%) |
| >4 sites | 14 (64%) |
| Nodal size | |
| ≥5 cm | 11 (50%) |
| ≥7 cm | 6 (27%) |
| . | Overall . |
|---|---|
| N = 22 (%) . | |
| Median age, y (range) | 53.5 (36-81) |
| Sex | |
| Male | 15 (68%) |
| Female | 7 (32%) |
| Stage | |
| II | 1 (5%) |
| III | 4 (18%) |
| IV | 17 (77%) |
| WHO classification grade | |
| I/II | 16 (73%) |
| IIIa | 6 (27%) |
| ECOG performance score | |
| 0 | 13 (59%) |
| 1 | 9 (41%) |
| FLIPI score | |
| Low risk | 4 (18%) |
| Intermediate risk | 12 (55%) |
| High risk | 6 (27%) |
| Nodal sites | |
| ≤4 sites | 8 (36%) |
| >4 sites | 14 (64%) |
| Nodal size | |
| ≥5 cm | 11 (50%) |
| ≥7 cm | 6 (27%) |
ECOG, Eastern Cooperative Oncology Group; FLIPI, Follicular Lymphoma International Prognostic Index; WHO, World Health Organization.
Primary objective
Patients were treated at DL0 (n = 3), DL1 (n = 3), and DL2 (n = 16). There were no protocol-defined dose-limiting toxicities reported at any dose level. Therefore, DL2 was determined to be the MTD and RP2D: lenalidomide 20 mg days 1 to 21 for eighteen 28-day cycles, ibrutinib 560 mg daily until progression.
Adverse events
Hematologic and nonhematologic adverse events are displayed in Tables 2 and 3, respectively. The most common grade 1 and 2 hematologic adverse events were anemia (27%) and thrombocytopenia (23%); the most common grade 3 and 4 hematologic adverse event was neutropenia (18%). The most common all-grade nonhematologic adverse events were rash (82%), diarrhea (64%), and fatigue (59%). There were no incidences of bleeding, thrombosis, or tumor flare. Grade 3 nonhematologic adverse events included rash (36%), diarrhea (5%), febrile neutropenia (5%), atrial flutter (5%), and arthralgia (5%). There were no grade 4 nonhematologic adverse events reported. New malignancies were reported in 5 patients: recurrent squamous cell carcinoma of the skin, melanoma in situ in an unusual mole that was being monitored closely before enrollment, prostate cancer in a patient with a history of an elevated prostate-specific antigen, endometrial carcinoma, and a poorly differentiated carcinoma. All malignancies were diagnosed within the first year of therapy.
Hematologic adverse events
| . | All grades . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Neutropenia | 36% | 9% | 9% | 9% | 9% |
| Thrombocytopenia | 33% | 23% | 5% | 5% | 0% |
| Anemia | 32% | 27% | 0% | 5% | 0% |
| Lymphopenia | 23% | 18% | 0% | 5% | 0% |
| . | All grades . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Neutropenia | 36% | 9% | 9% | 9% | 9% |
| Thrombocytopenia | 33% | 23% | 5% | 5% | 0% |
| Anemia | 32% | 27% | 0% | 5% | 0% |
| Lymphopenia | 23% | 18% | 0% | 5% | 0% |
Nonhematologic adverse events
| . | All grades . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Rash | 82% | 41% | 5% | 36% | 0% |
| Diarrhea | 64% | 50% | 9% | 5% | 0% |
| Fatigue | 59% | 45% | 14% | 0% | 0% |
| Infusion-related reaction | 37% | 14% | 23% | 0% | 0% |
| Nausea | 32% | 32% | 0% | 0% | 0% |
| Infection | 28% | 5% | 23% | 0% | 0% |
| Neoplasms | 23% | 0% | 5% | 18% | 0% |
| Arthralgia | 19% | 14% | 0% | 5% | 0% |
| Hyperbilirubinemia | 19% | 14% | 5% | 0% | 0% |
| ALT increased | 19% | 14% | 5% | 0% | 0% |
| AST increased | 18% | 18% | 0% | 0% | 0% |
| Headache | 18% | 18% | 0% | 0% | 0% |
| Constipation | 14% | 14% | 0% | 0% | 0% |
| Edema | 14% | 9% | 5% | 0% | 0% |
| Fever | 14% | 5% | 9% | 0% | 0% |
| Creatinine increased | 9% | 9% | 0% | 0% | 0% |
| Neuropathy | 9% | 9% | 0% | 0% | 0% |
| Hyperuricemia | 9% | 9% | 0% | 0% | 0% |
| Atrial flutter | 5% | 0% | 0% | 5% | 0% |
| Periorbital edema | 5% | 0% | 0% | 5% | 0% |
| Febrile neutropenia | 5% | 0% | 0% | 5% | 0% |
| . | All grades . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Rash | 82% | 41% | 5% | 36% | 0% |
| Diarrhea | 64% | 50% | 9% | 5% | 0% |
| Fatigue | 59% | 45% | 14% | 0% | 0% |
| Infusion-related reaction | 37% | 14% | 23% | 0% | 0% |
| Nausea | 32% | 32% | 0% | 0% | 0% |
| Infection | 28% | 5% | 23% | 0% | 0% |
| Neoplasms | 23% | 0% | 5% | 18% | 0% |
| Arthralgia | 19% | 14% | 0% | 5% | 0% |
| Hyperbilirubinemia | 19% | 14% | 5% | 0% | 0% |
| ALT increased | 19% | 14% | 5% | 0% | 0% |
| AST increased | 18% | 18% | 0% | 0% | 0% |
| Headache | 18% | 18% | 0% | 0% | 0% |
| Constipation | 14% | 14% | 0% | 0% | 0% |
| Edema | 14% | 9% | 5% | 0% | 0% |
| Fever | 14% | 5% | 9% | 0% | 0% |
| Creatinine increased | 9% | 9% | 0% | 0% | 0% |
| Neuropathy | 9% | 9% | 0% | 0% | 0% |
| Hyperuricemia | 9% | 9% | 0% | 0% | 0% |
| Atrial flutter | 5% | 0% | 0% | 5% | 0% |
| Periorbital edema | 5% | 0% | 0% | 5% | 0% |
| Febrile neutropenia | 5% | 0% | 0% | 5% | 0% |
ALT, alanine transaminase; AST, aspartate transaminase.
A total of 18 of 22 patients developed a rash with the regimen. Rashes were primarily maculopapular in nature, with exception of 1 pustular rash. Grade 1 or 2 rash occurred in 46% of patients, whereas grade 3 rash occurred in 36% of patients. Onset was typically during cycle 1, but occurred as late as cycle 4. Grades 1 and 2 rash resolved spontaneously without dose modification. Grade 3 rashes occurred at each dose level (Table 4). Grade 3 rashes that occurred during cycle 1 resolved within 10 days and were therefore not considered dose-limiting toxicities. Patients were treated with acetaminophen, diphenhydramine, and/or oral corticosteroids, and required treatment delay and dose level reduction as per protocol guidelines. Sulfamethoxazole/trimethoprim, prescribed for a urinary tract infection, was considered to be the etiology of grade 3 rash in 1 patient. Discontinuation of the antibiotic resulted in complete resolution of the rash, and the patient did not require any dose modifications. Shortly after study commencement, given that rash was being observed commonly, allopurinol was only used per investigator discretion for patients felt to be at high risk for tumor lysis syndrome. Ten of the 11 patients who received allopurinol developed a rash; however, 8 of the 11 patients who did not receive allopurinol also developed a rash. Two patients withdrew from the study because of persistent rashes that occurred after cycle 1. One of these patients underwent a biopsy of the rash, which showed findings consistent with a drug-induced erythrodermic hypersensitivity reaction, characterized by lymphocytic infiltration and scattered eosinophils.
Incidence of rash by DL
| . | All patients (n = 22) . | DL 0 (n = 3) . | DL 1 (n = 3) . | DL 2 (n = 16) . |
|---|---|---|---|---|
| All grades | 82% | 100% | 67% | 81% |
| Grade 1/2 | 46% | 67% | 33% | 44% |
| Grade 3/4 | 36% | 33% | 33% | 38% |
| . | All patients (n = 22) . | DL 0 (n = 3) . | DL 1 (n = 3) . | DL 2 (n = 16) . |
|---|---|---|---|---|
| All grades | 82% | 100% | 67% | 81% |
| Grade 1/2 | 46% | 67% | 33% | 44% |
| Grade 3/4 | 36% | 33% | 33% | 38% |
Definitions based on NCI CTCAE criteria, version 4.03: Grade 1: <10% body surface area (BSA) involved; Grade 2: 10-30% BSA involved, limiting instrumental activities of daily living (ADLs); Grade 3: >30% BSA involved, limiting self-care ADLs).
Treatment modifications
Twenty patients completed 6 cycles of rituximab, 10 patients completed 18 cycles of lenalidomide, and 9 patients continued to receive single-agent ibrutinib. The median time patients received ibrutinib monotherapy was 8 cycles (3-15+). Patients received 90% of the expected dose intensity of rituximab, 64% of lenalidomide, and 93% of ibrutinib. The dose intensity of rituximab and lenalidomide was calculated based on the planned duration of treatment of each agent. Because ibrutinib was to be administered indefinitely, the dose intensity of ibrutinib was calculated based on the number of cycles the patient received therapy in the study.
Eleven patients (50%) from all dose levels required dose reduction because of adverse events: rash (n = 7, 32%), neutropenia (n = 3, 14%), thrombocytopenia (n = 1, 5%). Six patients discontinued therapy because of adverse events including grade 3 rash (n = 2, 9%), grade 3 atrial flutter (n = 1, 5%), grade 3 diarrhea (n = 1, 5%), hypertension (n = 1, 5%), and depression (n = 1, 5%). The patients who developed an endometrial carcinoma and a poorly differentiated carcinoma required alternative interventions for their newly diagnosed malignancies, prompting study withdrawal.
Pharmacokinetics
Pharmacokinetic analyses were performed on plasma ibrutinib and its most potent metabolite, PCI-45227 concentrations, from data available in as many as 7 patients: DL0 (n = 2), DL1 (n = 3), DL2 (n = 2). Ibrutinib demonstrated rapid absorption with a median time to maximum concentration (Tmax) of 1 hour (range, 1-4). The median Cmax was 98.2 ng/mL (range, 15.4-354.0) and median AUC (0-24) was 669.5 ng.h/mL (range, 191.3-2072.2). The median elimination half-life was 6.9 hours (range, 4.1-8.9). There was minimal accumulation of ibrutinib by day 15. The R factor (based on AUC) was a maximum 1.3. The PCI-45227/ibrutinib AUC(0-24) ratio at steady state ranged from 0.9 to 3.1. Ibrutinib pharmacokinetic disposition when co-administered with lenalidomide and rituximab was consistent with that described for ibrutinib monotherapy at the doses studied.14,15
Efficacy
The ORR for the entire cohort of patients was 95%. Thirty-six percent (n = 8) of patients achieved CR (Table 5). An additional 7 patients (32%) experienced resolution of hypermetabolic activity on PET-CT consistent with a CR, but did not undergo a confirmatory bone marrow biopsy to rule out residual marrow involvement. All of the patients with pretreatment marrow involvement who achieved PET negativity and underwent trephine biopsy were found to have resolution of disease (n = 4). The response rates at the RP2D were similar: ORR 94% and CR 38%. The ORR by FLIPI risk group were 100% in low (n = 4), 100% in intermediate (n = 12), and 83% in high (n = 5). One high-risk patient maintained stable disease with the regimen. The median time to first response was 2.3 months (1.9-11.1), and the median time to best response was 5.5 months (1.9-25.1). Two patients chose to withdraw from the study after achieving CR. One remains in CR 9 months after discontinuing therapy, and the other was lost to follow-up.
Response rates by DL
| Response . | All patients (n = 22) . | DL 0 (n = 3) . | DL 1 (n = 3) . | DL 2 (n = 16) . |
|---|---|---|---|---|
| ORR | 95% | 100% | 100% | 94% |
| CR | 36% | 33% | 33% | 38% |
| PR | 59% | 67% | 67% | 56% |
| SD | 5% | 0 | 0 | 6% |
| Response . | All patients (n = 22) . | DL 0 (n = 3) . | DL 1 (n = 3) . | DL 2 (n = 16) . |
|---|---|---|---|---|
| ORR | 95% | 100% | 100% | 94% |
| CR | 36% | 33% | 33% | 38% |
| PR | 59% | 67% | 67% | 56% |
| SD | 5% | 0 | 0 | 6% |
CR, complete response; ORR, overall response rate; PR, partial response; SD, stable disease.
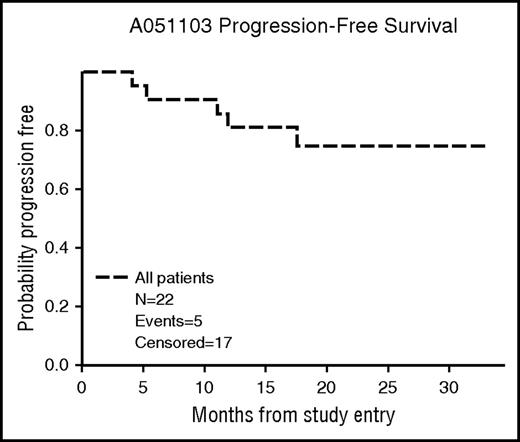
At a median follow-up of 19.2 months (2.3-27.1), the 12-month PFS was 80% (95% confidence interval [CI], 57%-92%) (Figure 2). Four patients progressed while receiving treatment during cycles 6, 12, 17, and 18. One patient progressed during cycle 3 during a 2-week treatment delay for grade 3 rash. One patient progressed 1 month after withdrawal from the study after cycle 13 for a diagnosis of endometrial carcinoma. None of the 8 other patients who discontinued therapy early have experienced a progression of follicular lymphoma. The patient found to have a metastatic poorly differentiated carcinoma died 1 month after study withdrawal. The remaining patients were still alive at the time of the final analysis.
Discussion
Alliance A051103 is the first clinical investigation of the combination of rituximab, lenalidomide, and ibrutinib. This single-arm, phase 1 trial expanded upon our prior experience with the rituximab-lenalidomide doublet in follicular lymphoma (CALGB 50401, CALGB 50803) by incorporating the use of a third distinct novel agent, ibrutinib. A051103 implemented similar inclusion criteria to previous frontline chemoimmunotherapy studies (FOLL05, PRIMA, NHL-2, BRIGHT), including advanced-stage patients, all FLIPI prognostic groups, and a clinical indication for treatment. Although a significantly smaller study, the triplet demonstrated efficacy comparable with traditional cytotoxic regimens (ORR 95%, CR 36%).1-4,6,7 Although the CR rate appeared lower than with previously reported regimens, nearly 50% of the patients who achieved PET negativity did not undergo confirmatory bone marrow biopsy and were not included in the reported value. Because all of the patients who had a follow-up biopsy per protocol were noted to have resolution of marrow involvement, it is possible that the true CR is as high as 68%. Responses were independent of FLIPI score and bulky disease, further supporting the consideration of biological regimens for the treatment of follicular lymphoma.
Although there were no protocol-defined, dose-limiting adverse events, the regimen was associated with clinically significant rash (all grades, 82%; grade 3, 36%). Rash occurred more frequently than reported with rituximab-lenalidomide (40%-58%, 7%-8%) or rituximab-ibrutinib (27%, 5%) in upfront follicular lymphoma or single-agent ibrutinib (25%, 3%-4%) and lenalidomide-ibrutinib (32%, 14%) in relapsed B-cell malignancies.6,7,15-17 Although line of therapy and disease histology may have affected the incidence and degree of rash, severe rash occurred at all dose levels with the triplet in this setting, prompting dose reduction in 7 patients and withdrawal from study in 2. One of the limitations of the study was the mandated reduction in dose per protocol, which may have affected depth of response. The depth of response, however, cannot be accurately evaluated because 7 patients who achieved a PET negativity did not undergo a confirmatory bone marrow biopsy. It may have been possible for at least some patients to continue at the same dose level with supportive care as has previously been demonstrated with single-agent lenalidomide. Although there is a short median follow-up, the durability of the regimen does not appear to be significantly compromised by the dose modifications thus far.
Confounding factors including concomitant medications were analyzed as potential etiologies for rash. Allopurinol was considered to be a culprit; however, a similar proportion of the patients who did not receive it also developed a rash. Aside from the use of a sulfa-based antibiotic in one patient, there were no other identifiable causes. The interaction between the monoclonal antibody, immunomodulatory agent, and BTK inhibitor appears to have triggered an undefined immune reaction, specific to follicular lymphoma. Preliminary unpublished data from a phase 1 study of the triplet in relapsed and refractory CLL do not suggest the same dermatologic toxicity profile (C.S.U., unpublished data; NCT02160015). Interestingly, the addition of a PI3 kinase antagonist, idelalisib, to rituximab-lenalidomide resulted in a higher degree of immune activation as is evident by 2 Alliance studies in relapsed follicular lymphoma and mantle cell lymphoma (A051201, A051202). Both trials were terminated early because of the development of an adverse event similar to a cytokine release syndrome, characterized by fever, hypotension, and rash.18 Preclinical studies of the combination of the 3 agents in this study had not been performed. Greater investigation into the effect of biological combinations on the malignant lymphocyte, tumor microenvironment, and additional targets, is necessary in understanding these types of outcomes. Given the significantly increased financial cost of this and similar multitargeted novel agent regimens compared with chemoimmunotherapy, strong scientific rationale for future studies is vital.
Compared with the frontline CALGB 50803, there appeared to be a greater incidence of grades 1-4 diarrhea (59% vs 33%) and arthralgia (19% vs 0), both of which are known adverse effects of ibrutinib. One patient withdrew from the study because of a persistent grade 3 diarrhea, which developed 2 years after study enrollment, suggesting that delayed toxicity can be seen with BCR antagonists. Similarly, there were a higher number of new malignancies (5 vs 0) with shorter follow-up. Although 3 patients had risk factors and all diagnoses were identified within a year of initiation of therapy, a potential correlation between the regimen and an accelerated development of malignancy remains possible. There were fewer thrombotic events than in CALGB 50803 (0 vs 3) or the relapsed population in CALGB 50401 (0 vs 2), which may have been related to the antiplatelet properties of aspirin and ibrutinib or merely smaller sample size. Notably, there were no bleeding events either, which may reflect the thrombogenic nature of lenalidomide.
Response data at dose level 2 appeared similar to CALGB 50803, but an accurate comparison of PFS is not yet possible given the shorter median follow-up. Other limitations of the study include small sample size and the lack of repeat bone marrow biopsies in some patients to confirm CR. Regardless, an improvement in clinical benefit with the additional third agent was not apparent. Increased toxicity and required dose modifications, including a 50% early discontinuance rate, is unacceptable for a frontline follicular lymphoma regimen. Although further investigation of the triplet seems unwarranted in this setting, the rituximab-lenalidomide combination remains a promising option. The RELEVANCE study will ultimately establish whether the biological doublet has a role as a frontline regimen for follicular lymphoma, and the AUGMENT study is evaluating this approach in the relapsed population. Ibrutinib may have a place in the relapsed setting as a single agent or in combination with another biological agent; however, better predictive markers are necessary given modest single-agent response rates in this population. As the treatment algorithm for follicular lymphoma continues to evolve, the rational incorporation of biological therapies is crucial in tailoring a safe, effective regimen for each patient.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Cancer Therapy Evaluation Program, Pharmacyclics LLC, and Celgene Corporation.
This study was supported by the National Cancer Institute (NCI) of the National Institutes of Health under award numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology); U10CA180833, U10CA180836, U10CA180838, U10CA180850, U10CA180854, U10CA180866, and U10CA180867. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The following institutions participated in this study (principal investigators and NCI grants in parentheses): Dana-Farber/Partners CancerCare LAPS, Boston, MA (Harold Burstein; 5U10CA180867); MedStar Georgetown University Hospital, Washington, DC (B.D.C.); Ohio State University Comprehensive Cancer Center LAPS, Columbus, OH (Richard Goldberg; U10CA180850); Roswell Park Cancer Institute LAPS, Buffalo, NY (E.L.; 5U10CA180866); UNC Lineberger Comprehensive Cancer Center LAPS, Chapel Hill, NC (Thomas Shea; 5U10CA180838); University of Chicago Comprehensive Cancer Center LAPS, Chicago, IL (Hedy Kindler; 5U10CA180836); and Weill Medical College of Cornell University, New York, NY (Scott Tagawa).
Authorship
Contribution: C.S.U., S.-H.J., J.P.L., S. M. Smith, L.D.L., and B.D.C. conceived and designed the study; C.S.U., B.P., J.P.L., L.D.L., N.L.B., and B.D.C. collected and assembled the data; and all authors analyzed and interpreted data, wrote the manuscript, and gave final approval of the manuscript.
Conflict-of-interest disclosure: C.S.U. has consulted for Pharmacyclics and Genentech. P.M. has consulted for Janssen, Celgene, and Novartis and received honoraria from Celgene. S.I.P. has traveled for Janssen. K.A.B. has received research funding from Cephalon, Celgene, Pharmacyclics, and Janssen. S. M. Smith has consulted for Celgene and Pharmacyclics. M.S.D. has consulted for Pharmacyclics, AbbVie, Janssen, and Celgene; has been a member of the Board of Directors or advisory committees for Pharmacyclics and Genentech; and received research funding from Pharmacyclics and Genentech. J.P.L. has consulted for Genentech and Celgene. N.L.B. has received research funding from Janssen, Pharmacyclics, Genentech, and Celegene. B.D.C. has consulted for and received research funding for Roche/Genentech, Celgene, and Pharmacyclics. L.D.L. has received research funding for industry-sponsored clinical trials from Novartis, GlaxoSmithKline, and AbbVie. The remaining authors declare no competing financial interests.
The current affiliation for M.C. is Celgene Corporation, Summit, NJ.
Correspondence: Chaitra S. Ujjani, 3800 Reservoir Rd NW, Washington, DC 20007; e-mail: csu@georgetown.edu.