In this issue of Blood, Kuhns et al1 describe the probable molecular and genetic basis for a disease that has mystified investigators for over 45 years, the lazy leukocyte syndrome. This disorder is characterized by recurrent infections, stomatitis, a low neutrophil count, and impaired neutrophil motility,2-5 and these abnormalities can now be explained by mutations in actin-interacting protein 1 (Aip1).1 This natural experiment provides exciting new insights into how neutrophils and other nonmuscle cells regulate their actin filament architecture to change shape and migrate.
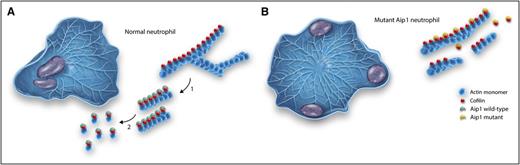
Actin-based motility in a normal and Aip-1 mutant neutrophil. (A) A normal neutrophil polarized after exposure to a chemoattractant (placed in the top right corner). The lamellipodia to the right is filled with dendritic (branched) networks of actin filaments. The actin filaments are magnified to the right. In step 1, cofilin binds to the actin filaments and breaks apart the dendritic networks, leaving long actin filaments. In step 2, activation by Aip1 results in efficient severing and complete disassembly of actin filaments. (B) A mutant Aip1 neutrophil. A dense network of actin filaments is found throughout the cell, preventing effective polarization or directional amoeboid movement. The nuclei are pushed to the periphery of the cell. Note in the magnified view that the actin filaments remain intact. The amount of mutant Aip1 protein is decreased and the reduced amounts of mutant protein fail to activate cofilin. Professional illustration by Somersault18:24.
Actin-based motility in a normal and Aip-1 mutant neutrophil. (A) A normal neutrophil polarized after exposure to a chemoattractant (placed in the top right corner). The lamellipodia to the right is filled with dendritic (branched) networks of actin filaments. The actin filaments are magnified to the right. In step 1, cofilin binds to the actin filaments and breaks apart the dendritic networks, leaving long actin filaments. In step 2, activation by Aip1 results in efficient severing and complete disassembly of actin filaments. (B) A mutant Aip1 neutrophil. A dense network of actin filaments is found throughout the cell, preventing effective polarization or directional amoeboid movement. The nuclei are pushed to the periphery of the cell. Note in the magnified view that the actin filaments remain intact. The amount of mutant Aip1 protein is decreased and the reduced amounts of mutant protein fail to activate cofilin. Professional illustration by Somersault18:24.
To fully understand the implications of their findings, the reader needs to understand a few fundamental facts about actin. Actin is present in high concentrations (∼200 µM) in the cytoplasm of neutrophils, and this protein exists in 2 states: as monomers that can readily diffuse throughout the cytoplasm, and as actin filaments that can form branched networks that support the formation of lamellipodia, broad protrusions seen at the front of neutrophils as they migrate (see figure panel A). Great progress has been made in understanding how actin regulatory proteins and specific signal transduction pathways induce actin assembly. However, in order to change shape and produce amoeboid movement, actin filaments must also be quickly disassembled, and at the present time, our understanding of these mechanisms is more rudimentary. Actin depolymerizing protein (ADF; also called cofilin) has been isolated and shown to bind to the sides of actin filaments and to destabilize monomer–monomer interactions within the filament, causing actin filaments to break apart or undergo severing.6 The ability of ADF/cofilin to sever actin filaments varies depending on the stoichiometry of binding. When actin filaments are saturated with ADF/cofilin, severing is minimal and filaments are stabilized7 ; however, in living cells, despite the high concentrations of ADF/cofilin, actin filaments undergo rapid severing.8
Several proteins have been identified that may account for this activity, including coronins,9 Srv2/cyclase-associated protein,10 and Aip1.8 In the absence of specific mutations and knockout experiments, the relative importance of each of these proteins in potentiating the severing of activity of ADF/cofilin is difficult to assess. The 3 families with genetic mutations described by Kuhns et al now help to address this important question. Family members and patients all had mutations in the WDR1 gene that encodes for Aip1. The predicted amino acid changes were localized to the conserved regions of C-terminal and N-terminal regions of the β sheet or resulted in missense mutations that are predicted to result in breakdown of the expressed protein. It is likely that all of these mutations significantly reduce the normal activity Aip1 to potentiate the severing activity of ADF/cofilin. The marked increase in neutrophil actin filament content, failure of these cells to form a normal polar structures with lamellipodia at the front, and the marked slowing of cell motility in response to chemoattractants all emphasize the central role of Aip1 in potentiating actin filament recycling and emphasize the vital role of Aip1 in supporting neutrophil motility (see figure panel B). These exciting findings emphasize that the study of genetic diseases, combined with basic cell biology and biochemistry, can provide the fullest understanding of structure–function relationships and promises to provide future targets for controlling inflammation and improving host defense.
Conflict-of-interest disclosure: The author declares no competing financial interests.