Key Points
Mutations in Aip1, encoded by WDR1, alter regulation of the neutrophil cytoskeleton, causing neutrophil dysfunction.
Abstract
Cell motility, division, and structural integrity depend on dynamic remodeling of the cellular cytoskeleton, which is regulated in part by actin polymerization and depolymerization. In 3 families, we identified 4 children with recurrent infections and varying clinical manifestations including mild neutropenia, impaired wound healing, severe stomatitis with oral stenosis, and death. All patients studied had similar distinctive neutrophil herniation of the nuclear lobes and agranular regions within the cytosol. Chemotaxis and chemokinesis were markedly impaired, but staphylococcal killing was normal, and neutrophil oxidative burst was increased both basally and on stimulation. Neutrophil spreading on glass and cell polarization were also impaired. Neutrophil F-actin was elevated fourfold, suggesting an abnormality in F-actin regulation. Two-dimensional differential in-gel electrophoresis identified abnormal actin-interacting protein 1 (Aip1), encoded by WDR1, in patient samples. Biallelic mutations in WDR1 affecting distinct antiparallel β-strands of Aip1 were identified in all patients. It has been previously reported that Aip1 regulates cofilin-mediated actin depolymerization, which is required for normal neutrophil function. Heterozygous mutations in clinically normal relatives confirmed that WDR1 deficiency is autosomal recessive. Allogeneic stem cell transplantation corrected the immunologic defect in 1 patient. Mutations in WDR1 affect neutrophil morphology, motility, and function, causing a novel primary immunodeficiency.
Introduction
Normal neutrophil function depends on rapid dynamic remodeling of filamentous actin. Whereas in muscle cells actin exists predominantly as a filamentous polymer (F-actin), in nonmuscle cells, at least half of the actin is retained as a globular monomer (G-actin). A thin submembranous cortical layer of F-actin often maintains structural integrity and regulates exocytosis.1,2 Regulation of cytoskeletal dynamics is complex and depends on a variety of actin binding proteins; some are involved in promoting F-actin polymerization, whereas others promote severing of actin to shorter actin oligomers or G-actin monomers. Profilin binds 1:1 to G-actin, preventing actin polymerization and reserving a pool of monomers for filament assembly.3 In contrast, cofilin (actin depolymerizing factor) binds F-actin and catalyzes the severing of actin filaments into shorter actin oligomers.4,5
There have been relatively few reports of patients with neutrophil defects involving the actin cytoskeleton. “Lazy-leukocyte syndrome” described a condition characterized by recurrent infections, stomatitis, neutropenia, abnormal neutrophil random (chemokinesis) and directed (chemotaxis) migration, and defective neutrophil mobilization.6-8 Despite clear demonstration of abnormal actin polymerization and impaired chemotaxis, no genetic etiology was identified. Nunoi et al identified a patient with a mutation in β-actin that reduced the binding of profilin to actin, resulting in an immunodeficiency characterized by reduced neutrophil chemotaxis and superoxide production. These cases confirmed that actin dysfunction could cause neutrophil defects of variable clinical expression.9 Patient II.2.2 in this report was previously10 described as a child with severe stomatitis who had abnormal chemotaxis and chemokinesis, as well as herniation of neutrophil nuclear lobes; she died of overwhelming varicella infection at age 8.10
Here we describe studies of additional families with life-threatening infections, impaired chemotaxis and chemokinesis, and neutrophil nuclear lobe herniation. Through biochemical and functional approaches, we found abnormalities in actin-interacting protein 1 (Aip1), encoded by the gene WD repeat protein 1 (WDR1), identifying a novel immunodeficiency.
Methods
Case reports
Family I.
Two Qatari sisters from a consanguineous union were referred in 2005 for frequent severe skin and mucosal ulcerations, recurrent upper and lower respiratory tract infections, and mild neutropenia (absolute neutrophil counts, 1800-2000/μL in the absence of active infection or granulocyte colony-stimulating factor stimulation) (family I; Figure 1A). By age 7, patient I.2.3 had oral stenosis severe enough to require gastrostomy tube feeding (Figure 1B). Patient 1.2.3 had multiple infections, including pneumonias with Staphylococcus aureus and Haemophilus influenzae and urinary tract infection with Escherichia coli and Enterococcus. A twin sister (patient I.2.4) reportedly died of sepsis in Qatar at age 3 following a diarrheal illness. An older sister (patient 1.2.2) had recurrent cutaneous and pulmonary infections that responded to oral antibiotic therapy directed at gram positives, but no pathogens were identified. A scalp biopsy from an area of alopecia in 1.2.2 showed only a chronically inflamed superficial scar. Patient I.2.3 underwent successful stem cell transplantation at age 9 from her unaffected sibling, I.2.1.
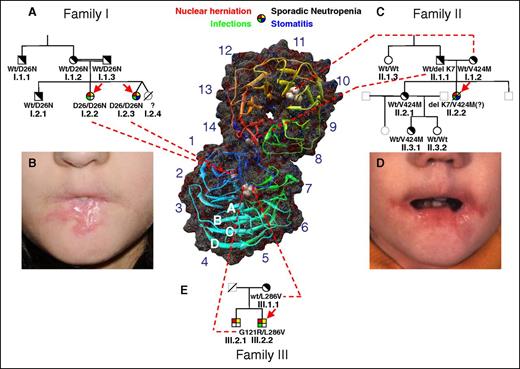
Pedigrees of families. (A) Family I. The 2 probands (I.2.2 and I.2.3 indicated by red arrows) were studied on several occasions with reproducible neutrophil abnormalities measured. The deceased twin I.2.4 is presumed to have been affected. The probands are homozygous for D26N. No abnormalities were noted in the neutrophils of the heterozygous parents, brother, or maternal uncle. Clinical features of affected individuals are designated by the 4-color filled symbol at the top. Heterozygous carriers are indicated by diagonally filled symbols. Wild-type individuals are indicated by open symbols. Open symbols in gray represent untested family members. A symbol with a diagonal line indicates a deceased family member. (B) Stomatitis with severe oral stenosis in patient I.2.3. (C) Family II. Patient II.2.2 was originally reported in 1978,10 at which time many studies were performed as reported. Because no DNA from her was recoverable, her genotype was inferred from her parents and sibling. She is presumed to have been compound heterozygous for delK7 and V424M. (D) Stomatitis in patient II.2.2. (E) Family 3. Patient III.2.2 was compound heterozygous for L286V and G121R, which are presumed to reside on different alleles by virtue of only 1 allele being found in the mother. The clinically affected brother, III.2.1, was not available for genetic study. (F) Molecular modeling of human Aip1 based on S cerevisiae Aip1/UNC78 shows a dual β-propeller, comprised of an N-terminal propeller (lower) and a C-terminal propeller (upper). Each propeller consists of 7 blades, labeled 1 to 7 in the N-terminal propeller and 8 to 14 in the C-terminal peptide. Each blade consists of a twisted β-sheet comprised of 4 antiparallel strands (A-D). The N terminus of the peptide (shown in blue) traverses the hinge region and forms the D strand of blade 14 (shown in red), conferring rigidity to the open “clamshell” structure. Each of the mutations identified have been superimposed on the images.
Pedigrees of families. (A) Family I. The 2 probands (I.2.2 and I.2.3 indicated by red arrows) were studied on several occasions with reproducible neutrophil abnormalities measured. The deceased twin I.2.4 is presumed to have been affected. The probands are homozygous for D26N. No abnormalities were noted in the neutrophils of the heterozygous parents, brother, or maternal uncle. Clinical features of affected individuals are designated by the 4-color filled symbol at the top. Heterozygous carriers are indicated by diagonally filled symbols. Wild-type individuals are indicated by open symbols. Open symbols in gray represent untested family members. A symbol with a diagonal line indicates a deceased family member. (B) Stomatitis with severe oral stenosis in patient I.2.3. (C) Family II. Patient II.2.2 was originally reported in 1978,10 at which time many studies were performed as reported. Because no DNA from her was recoverable, her genotype was inferred from her parents and sibling. She is presumed to have been compound heterozygous for delK7 and V424M. (D) Stomatitis in patient II.2.2. (E) Family 3. Patient III.2.2 was compound heterozygous for L286V and G121R, which are presumed to reside on different alleles by virtue of only 1 allele being found in the mother. The clinically affected brother, III.2.1, was not available for genetic study. (F) Molecular modeling of human Aip1 based on S cerevisiae Aip1/UNC78 shows a dual β-propeller, comprised of an N-terminal propeller (lower) and a C-terminal propeller (upper). Each propeller consists of 7 blades, labeled 1 to 7 in the N-terminal propeller and 8 to 14 in the C-terminal peptide. Each blade consists of a twisted β-sheet comprised of 4 antiparallel strands (A-D). The N terminus of the peptide (shown in blue) traverses the hinge region and forms the D strand of blade 14 (shown in red), conferring rigidity to the open “clamshell” structure. Each of the mutations identified have been superimposed on the images.
Family II.
A 7-year-old girl from a nonconsanguineous union was referred in 1976 (Figure 1C) for recurrent infections, neutropenia, and stomatitis (Figure 1D), with abnormal neutrophil morphology and defective random and directed locomotion. She had multiple pyogenic infections, including pneumococcal sinusitis, H influenzae otitis media, and 3 episodes of Streptococcus pneumoniae lobar pneumonia. In addition, pathogenic staphylococci and streptococci were repeatedly cultured from small areas of cutaneous inflammation. Recurrent otitis and sinusitis could not be controlled sufficiently to prevent hearing loss or oral stenosis. She died at age 8 from disseminated varicella infection.10
Family III.
Two brothers from a nonconsanguineous union were referred in 1986 (Figure 1E) with histories of recurrent infections and mild neutropenia. Both had abnormal neutrophil morphology. In 2011, patient III.2.2 continued to have abnormal neutrophil morphology.
Assays of neutrophil function
Heparinized bloods from both normal subjects and patients were drawn after written consent was obtained under the Frederick Research Donor Program protocol OH99-C-N046 and National Institutes of Health (NIH) protocols 99-CC-0168 and 93-I-0119. Neutrophils and peripheral blood mononuclear cells (PBMCs) were harvested from diluted blood by discontinuous gradient centrifugation (500g for 30 minutes at room temperature) on a cushion of Ficoll-Paque (GE Healthcare). PBMCs were harvested from the top of the Ficoll-Paque cushion. The neutrophil-enriched erythrocyte pellet was resuspended with an equal volume of 3.0% dextran (molecular weight, 300 000; MP Biomedical), and the erythrocytes were sedimented at 1g for 30 minutes. Remaining contaminating erythrocytes were removed by sequential hypotonic lysis (equal volumes of 0.2% saline followed by 1.6% saline within 30 seconds to restore isotonicity). The final preparation of neutrophils was >95% pure with 4% eosinophils and <1% monocytes and lymphocytes as assessed by differential staining.
Isolated neutrophils (2.0 × 104) were centrifuged onto glass at 100g in a cytocentrifuge and stained with Diff-Quick (Harleco, Gibbstown, NJ), as were blood smears. For electron micrographs, 2.5 × 106 cells were pelleted at 300g and overlayed with 2% glutaraldehyde in cacodylate buffer.
Cell spreading was monitored after isolated neutrophils (20 000 cells/20 µL) were added to a microscope slide, immediately covered with a coverslip, and viewed at ×1000. Digital images were captured every 5 seconds for 10 minutes using an Infinity2-1 digital camera (Lumenera Corp, Ottawa, CA) attached to a microscope (Model BX50; Olympus Corp, Center Valley, PA). Images were analyzed using Infinity Analyze software (Lumenera Corporation, Ottawa, ON, Canada) to determine the temporal changes in the perimeter and area of individual cells as they settled and spread on the microscope slide.
Formyl-methionyl-leucyl-phenylalanine (fMLF, 5 × 10−9 M)–induced changes in neutrophil morphology were assessed using the forward and right angle light scattering channels of a BD FACSCanto II Analyzer.
Neutrophil chemotaxis was measured using an EZ-TAXIScan (Effector Cell Institute, Tokyo, Japan). Isolated neutrophils (1.0 µL of 5 × 106/mL) were added to the “Cell” well of the EZ-TAXIScan and 1.0 µL of either buffer or fMLF (5 × 10−8 M) was added to the opposing “Chemoattractant” well. Digital images of the migrating PMNs were captured every 2.5 minutes for 1 hour. Images were converted to stacks using the ImageJ software (version 1.46r; NIH). Ten randomly selected cells were electronically traced using the ImageJ plug-in, MTrackJ. The paths of the migrating cells were plotted with the position of each cell at t = 0 anchored at the origin. Using the coordinates of the individual cells in each image, the distance that each cell migrated and the average velocity were calculated using the distance formula.
Superoxide production was measured by the superoxide dismutase-inhibitable reduction of ferricytochrome c. Neutrophils, suspended in Hanks balanced salt solution (HBSS; 1 × 106/mL) containing 100 µM ferricytochrome c, were incubated at 37°C for 15 minutes in the presence of buffer (basal), fMLF (10−7 M), phorbol myristate acetate (PMA) (100 ng/mL), cytochalasin B (5 µg/mL), opsonized zymosan (1 mg/mL), or the combination of cytochalasin B + opsonized zymosan. The reaction was stopped at 4°C, and the supernatant fluid was harvested after centrifuging. An identically treated tube containing superoxide dismutase (100 µg/mL) served as the blank for each set of conditions. The reduction of ferricytochrome c was assayed with an analytic wavelength of 549.5 nm and background wavelengths at the isosbestic points of 541 and 556 nm. The data were converted to nanomoles of  cells using a micromolar extinction coefficient of 0.0211.
cells using a micromolar extinction coefficient of 0.0211.
The staphylococcidal activity of neutrophils, suspended at 5 × 106 cells/mL in HBSS with divalent cations and 10% fresh frozen AB serum, was assessed using S aureus strain 502A at target:effector ratios of 2:1 and 8:1. After incubating 20, 45, and 90 minutes, the surviving bacteria in the cell suspension were cultured on agar pour plates. Colony-forming units were enumerated using Image-Pro Plus (MediaCybernetics, Bethesda, MD).
Neutrophil adherence was determined as binding of calcein acetomethoxy-loaded (Life Technologies, Grand Island, NY) neutrophils to plastic after stimulation with phorbol myristate acetate (100 ng/mL; Sigma-Aldrich Corp, St Louis, MO) using a fluorescence microplate reader (Gemini EM; Molecular Devices, Sunnyvale, CA).
Neutrophil degranulation was assessed by stimulation for 15 minutes at 37° with either PMA (100 ng/mL) or with fMLF (0.1 µM) plus cytochalasin B (5 µg/mL). The levels of lactoferrin, gelatinase (R&D Systems, Minneapolis, MN), and myeloperoxidase (MesoScale Discovery, Gaithersburg, MD) in the extracellular fluids and the cell pellets were determined with commercial enzyme-linked immunoassays according to manufacturers’ instructions.
To visualize F-actin by confocal microscopy (LSM510; Zeiss, Jena, Germany), neutrophils (2.0 × 104) were dried onto glass slides, permeabilized with 0.1% TritonX-100, and stained with 5 units Alexa Fluor 546 phalloidin (Life Technologies). Slides were counterstained with the nuclear stain, 4',6-diamidino-2-phenylindole (0.1 µg/mL). Alternatively, neutrophils (1.0 × 106/mL) were incubated for 10 minutes at 37°C with 3.7% formaldehyde, 1.25 U/mL Alexa Fluor 546 phalloidin, and 100 μg/mL lysophosphatidylcholine (Sigma-Aldrich), washed, and analyzed on a BD FACSCanto II Analyzer (BD Biosciences, San Jose, CA).
Two-dimensional differential gel electrophoresis of neutrophil lysates and identification of proteins by mass spectrometry
Five million diisopropyl-fluorophosphate–treated neutrophils were lysed (100 µL, 30 mM Tris-HCl, pH 8.8, 7 M urea, 2 M thiourea, and 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate). Protein was measured using Bio-Rad assay. For each sample, 30 µg protein was mixed with 1.0 µL diluted CyDye and kept in the dark on ice for 30 minutes. One microliter 10 mM lysine was added to each sample and incubated in the dark on ice for an additional 15 minutes to stop the reaction. Each Cy3-labeled patient sample was mixed with a Cy5-labeled normal sample. Proteins were first resolved by isoelectric focusing, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) orthogonally. The resulting gel was imaged for both fluorescent dyes, and the relative expression (ratio of the Cy dyes) of each protein spot was determined. Protein spots that were differentially expressed in both patient samples compared with the normal samples (significance criterion of P < .1) were selected for identification by mass spectrometry (Applied Biomics, Hayward, CA). Additional details of the methods are included in the supplemental Data, available on the Blood Web site.
Immunoblot analysis
Differential expression of neutrophil proteins was confirmed by electrophoresis of 75 µg neutrophil lysate on a 7.5% Bis-Tris SDS-PAGE gel, transfer to nitrocellulose, and probing with antibodies to cofilin (Cytoskeleton, Denver, CO), phospho-cofilin (Ser3) (Cell Signaling Technology, Danvers, MA), WDR1/Aip1 (Novus Biologicals, Littleton, CO), and actin (Abcam, Cambridge, MA).
Gene sequence analysis
Genomic DNA was isolated from neutrophils, PBMCs, or Epstein-Barr virus–transformed B cells using the Gentra Puregene kit (Qiagen, Valencia, CA). The ratio of absorbances (Abs260nm/Abs280nm) was used to assess the DNA purity of each sample and was generally >1.7; DNA concentrations were determined using a Nanodrop (Thermo Fisher Scientific, Waltham, MA). Specific details of the polymerase chain reaction assays and sequencing reactions are included in the supplemental Data.
Results
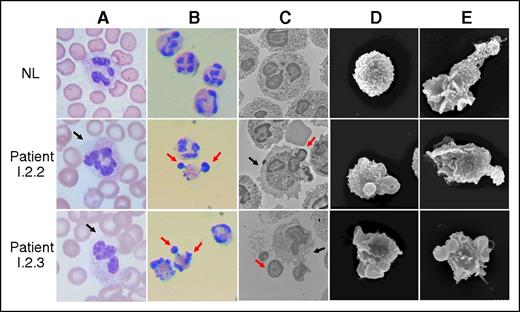
Peripheral blood smears from the patients in family I repeatedly showed neutrophil cytosolic regions devoid of granules (Figure 2, column A). Isolated neutrophils spread on glass showed distinctive herniation of nuclear lobes in 40% to 60% of the cells (Figure 2, column B). Nuclear abnormalities were only observed in patient neutrophils and eosinophils. Transmission electron micrographs confirmed the herniation of up to 3 nuclear lobes in both neutrophils and eosinophils, with retraction of cytoplasmic granules into the central region of the cells (Figure 2, column C). A thin region of cytosol and plasma membrane surrounded the herniated nuclear lobes, confirming their contiguity with the parent cell. Under basal conditions, normal neutrophils were spherical by scanning electron microscopy (Figure 2, column D), whereas patient neutrophils exhibited knob-like structures, presumed to be nuclear lobes. Treatment of neutrophils with the chemoattractant fMLF for 30 minutes normally induces cytoskeletal reorganization, leading to neutrophil polarization with a lamellipod on the leading edge and restriction of granules and nuclear lobes to the trailing uropod (Figure 2, column E). Neutrophil polarization and ruffling of the lamellipod were tracked temporally using the forward and right angle light scattering channels of a flow cytometer (Figure 3A). Patient neutrophils failed to polarize normally (Figures 2, column E, and 3A). In addition, neutrophils from patient I.2.3 failed to spread on glass normally (supplemental Movie 1) compared with a normal subject. Using image analysis to determine the temporal changes in the area of each cell, neutrophils from normal subjects spreading on glass exhibited a greater than twofold increase in area; neutrophils from patient I.2.3 and patient III.2.2 exhibited abnormal spreading on glass that was significantly different compared with neutrophils from normal subjects (supplemental Figure 1A-B).
Morphology of neutrophils. (A) Neutrophils from whole blood smears of a normal subject (NL, top row) and patients I.2.2 (middle row) and I.2.3 (bottom row) stained with Diff-Quik. The black arrows indicate regions of abnormally agranular cytoplasm. (B) Neutrophils prepared by Ficoll centrifugation, 3% dextran sedimentation, and sequential erythrocyte hypotonic lysis. Neutrophils were centrifuged onto slides at 100g for 10 minutes and then stained. Red arrows indicate herniated nuclear lobes. (C) Transmission electron micrographs of isolated neutrophils. Black arrows indicate areas of abnormally agranular cytoplasm; red arrows indicate herniated nuclear lobes. Overall, 40% to 60% of patient cells had abnormal morphology. (D) Scanning electron micrographs of isolated neutrophils, showing knob-like projections on patient neutrophils (magnification, ×6000). (E) Scanning electron micrographs of isolated neutrophils. Stimulation of normal cells with fMLF resulted in polarization with elongation of the cells with protruding lamellipodia and trailing uropod. Although neutrophils from patients I.2.2 and I.2.3 underwent some morphology change, they failed to exhibit the morphological changes of normal subjects.
Morphology of neutrophils. (A) Neutrophils from whole blood smears of a normal subject (NL, top row) and patients I.2.2 (middle row) and I.2.3 (bottom row) stained with Diff-Quik. The black arrows indicate regions of abnormally agranular cytoplasm. (B) Neutrophils prepared by Ficoll centrifugation, 3% dextran sedimentation, and sequential erythrocyte hypotonic lysis. Neutrophils were centrifuged onto slides at 100g for 10 minutes and then stained. Red arrows indicate herniated nuclear lobes. (C) Transmission electron micrographs of isolated neutrophils. Black arrows indicate areas of abnormally agranular cytoplasm; red arrows indicate herniated nuclear lobes. Overall, 40% to 60% of patient cells had abnormal morphology. (D) Scanning electron micrographs of isolated neutrophils, showing knob-like projections on patient neutrophils (magnification, ×6000). (E) Scanning electron micrographs of isolated neutrophils. Stimulation of normal cells with fMLF resulted in polarization with elongation of the cells with protruding lamellipodia and trailing uropod. Although neutrophils from patients I.2.2 and I.2.3 underwent some morphology change, they failed to exhibit the morphological changes of normal subjects.
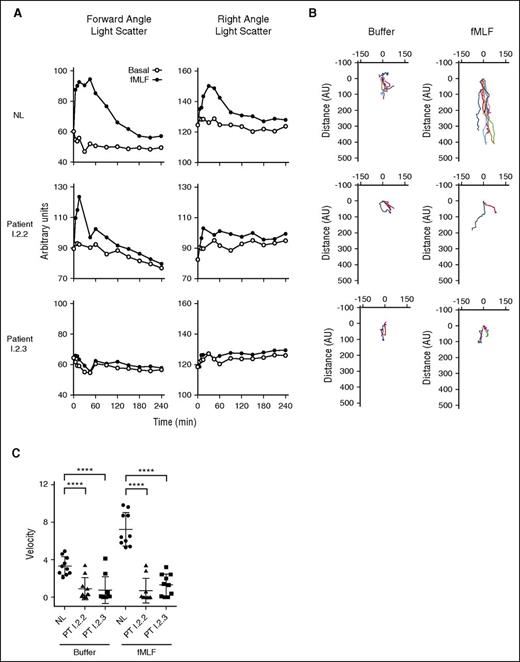
Patient neutrophil dysfunction. (A) (Left column) Temporal changes in forward angle light scatter (polarization) in normal neutrophils and neutrophils from patients I.2.2 and I.2.3 after treatment with buffer or fMLF (1 × 10−7 M). (Right column) Similar time course of changes in right angle light scatter (membrane ruffling). In B, neutrophils (1 μL of 2 × 106 cells/mL in HBSS with divalent cations) were added to the “Cell” well of EZ-TAXIScan and either buffer or fMLF was added to the “Chemoattractant” well. The cells were incubated for 60 minutes, and images were collected every 2.5 minutes. Ten randomly chosen cells were electronically traced using the acquired images and the paths of the cells plotted with the position at t = 0 anchored at the origin. (Left column) Random migration of neutrophils from a normal subject and from patients I.2.2 and I.2.3. (Right column) Directed migration in response to fMLF (1 × 10−8 M). (C) Scattergrams of the average velocities of the individual cells that were tracked in B. Note that the cells from both patients have a significant defect (analysis of variance, ****P < .001) in both their basal migration and a defect in their migration to the chemoattractant, fMLF.
Patient neutrophil dysfunction. (A) (Left column) Temporal changes in forward angle light scatter (polarization) in normal neutrophils and neutrophils from patients I.2.2 and I.2.3 after treatment with buffer or fMLF (1 × 10−7 M). (Right column) Similar time course of changes in right angle light scatter (membrane ruffling). In B, neutrophils (1 μL of 2 × 106 cells/mL in HBSS with divalent cations) were added to the “Cell” well of EZ-TAXIScan and either buffer or fMLF was added to the “Chemoattractant” well. The cells were incubated for 60 minutes, and images were collected every 2.5 minutes. Ten randomly chosen cells were electronically traced using the acquired images and the paths of the cells plotted with the position at t = 0 anchored at the origin. (Left column) Random migration of neutrophils from a normal subject and from patients I.2.2 and I.2.3. (Right column) Directed migration in response to fMLF (1 × 10−8 M). (C) Scattergrams of the average velocities of the individual cells that were tracked in B. Note that the cells from both patients have a significant defect (analysis of variance, ****P < .001) in both their basal migration and a defect in their migration to the chemoattractant, fMLF.
The neutrophils of patients I.2.2 and I.2.3 had increased spontaneous and fMLF- and opsonized zymosan-stimulated superoxide production (supplemental Figure 2) as shown previously for patient II.2.2.10 However, PMA-stimulated superoxide production was normal. Cytochalasin B is thought to bind to the barbed end of F-actin and prevent monomer addition, promoting depolymerization of F-actin.11 In normal neutrophils, cytochalasin B amplifies opsonized zymosan-induced superoxide production.12 However, patient neutrophils showed negligible superoxide augmentation with cytochalasin B, suggesting that their basal state mimicked that induced by cytochalasin B and reflected an underlying defect in actin kinetics (supplemental Figure 2). Total granule content of lactoferrin, myeloperoxidase, and gelatinase in patient neutrophils was normal, as was their release in response to PMA and fMLF + cytochalasin B (not shown). Patient neutrophils killed S aureus normally at 20 and 45 minutes, but exhibited a slight defect at 90 minutes regardless of multiplicity of infection (supplemental Figure 3).
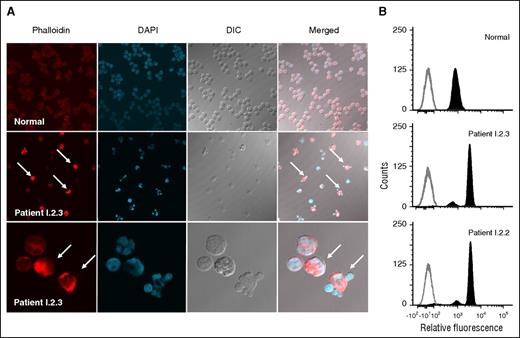
Neutrophil migration requires rapid rearrangement of actin. Tracking the movement of equal numbers of individual cells (EZ-TAXIScan) showed profoundly impaired random and fMLF-directed migration of neutrophils from patients I.2.2 and I.2.3 (Figure 3B-C). Actin in the resting neutrophil was stained with Alexa Fluor 546 phalloidin, a fluorescent probe that preferentially binds F-actin. Confocal microscopy showed that 50% to 90% of patient cells had increased phalloidin staining, indicating increased F-actin (Figure 4A). Taken together, these data indicated an abnormality in the abundance and localization of F-actin. Importantly, herniation of nuclear lobes colocalized with increased F-actin staining. Normal neutrophils stained with Alexa Fluor 546 phalloidin by flow cytometry showed only a single population of neutrophils (Figure 4A-B). In contrast, neutrophils from patients I.2.2 and I.2.3 showed only minor populations with normal phalloidin staining, whereas the majority of cells (∼85%) had fourfold more phalloidin staining than normal neutrophils (3520 and 3240 AU for patients I.2.2 and I.2.3, respectively, compared with 830 AU for the normal), confirming that patients had increased levels of F-actin (Figure 4B).
Abnormal neutrophil morphology and association with increased microfilaments. (A) Neutrophils from a normal subject (top row) and patient I.2.3 (middle row) were stained for F-actin (Alex Fluor 546 phalloidin), and the nuclei were stained with 4',6-diamidino-2-phenylindole. Fluorescence and differential interference contrast images are shown individually and merged. Neutrophils with herniated nuclear lobes are indicated by white arrows in the phalloidin-stained and merged images of patient I.2.3. A higher magnification (×1000) of neutrophils from patient I.2.3 is presented in the bottom row. (B) Neutrophil flow cytometric phalloidin staining. Unstained cells are shown in gray outline, whereas F-actin–containing cells labeled with Alex Fluor 546 phalloidin are shown in solid dark.
Abnormal neutrophil morphology and association with increased microfilaments. (A) Neutrophils from a normal subject (top row) and patient I.2.3 (middle row) were stained for F-actin (Alex Fluor 546 phalloidin), and the nuclei were stained with 4',6-diamidino-2-phenylindole. Fluorescence and differential interference contrast images are shown individually and merged. Neutrophils with herniated nuclear lobes are indicated by white arrows in the phalloidin-stained and merged images of patient I.2.3. A higher magnification (×1000) of neutrophils from patient I.2.3 is presented in the bottom row. (B) Neutrophil flow cytometric phalloidin staining. Unstained cells are shown in gray outline, whereas F-actin–containing cells labeled with Alex Fluor 546 phalloidin are shown in solid dark.
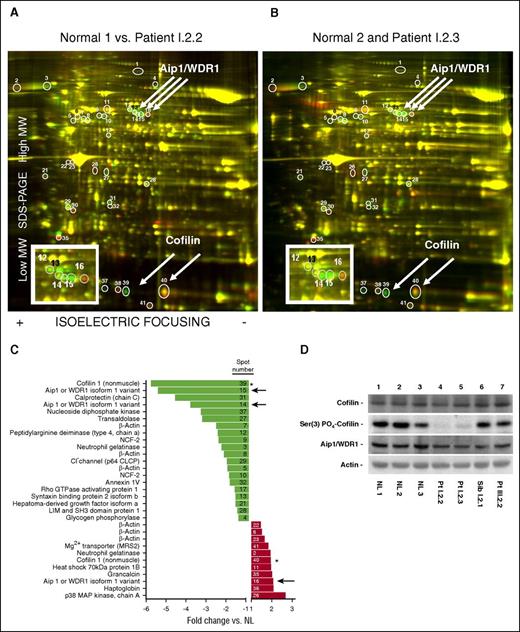
Because the disease phenotype was transmitted in a recessive pattern and appeared to be in neutrophil cytosol, we chose a proteomic approach searching for abnormal or missing proteins in our patients. Normal and patient neutrophil lysates were labeled and examined by 2D differential in-gel electrophoresis (Figure 5A-B). Thirty-one proteins were differentially detected in patient neutrophil lysates compared with normals (Figure 5C) including 5 β-actin variants, suggesting differential regulation of posttranslational modifications of actin in patient neutrophils. Two spots were identified as cofilin (one being more acidic and less abundant and the other more basic and abundant in patient neutrophils). However, total patient cofilin levels were normal (Figure 5D), suggesting that cofilin was differentially regulated by posttranslational modifications.
Two-dimensional differential in-gel electrophoresis of neutrophil lysates. (A) Lysates of normal and patient I.2.2 neutrophils were differentially labeled (with Cy3, green, and Cy5, red, respectively) and mixed together, and the proteins were resolved by 2D gel electrophoresis (isoelectric focusing horizontally, and SDS-PAGE vertically). The protein spots on the resolved proteins gels were imaged for the red and green dye fluorescence, respectively, and then the ratios of the images were determined. Green spots indicate increased protein expression in the lysate of neutrophils from the normal subject compared with the lysate from neutrophils of patient I.2.2; similarly, red spots indicate increased protein expression in the lysate of neutrophils from patient I.2.2. Convergence of spots resulting in yellow indicated equivalent protein expression in the lysates of neutrophils from both subjects. The spots that were differentially expressed are indicated by circles and identified by numbers. The identity of these differentially expressed spots was excised and determined by mass spectrometry. The inset is an enlargement of the region where Aip1 migrated. (B) Same analysis for normal compared with patient I.2.3. (C) Relative changes in the mean (normal vs patient) expression of proteins in neutrophil lysates. The number in each bar refers to the specific spot on the 2D gel. Note that both cofilin (*) and AIP1 (arrows) are represented in multiple spots. (D) Protein expression in neutrophil lysates from 3 normal subjects, patients I.2.2 and I.2.3, and their unaffected heterozygous sibling, I.2.1, and patient III.2.2 of family III. The immunoblots were probed with antibodies to Aip1, cofilin, phospho-cofilin Ser(3), and actin as indicated.
Two-dimensional differential in-gel electrophoresis of neutrophil lysates. (A) Lysates of normal and patient I.2.2 neutrophils were differentially labeled (with Cy3, green, and Cy5, red, respectively) and mixed together, and the proteins were resolved by 2D gel electrophoresis (isoelectric focusing horizontally, and SDS-PAGE vertically). The protein spots on the resolved proteins gels were imaged for the red and green dye fluorescence, respectively, and then the ratios of the images were determined. Green spots indicate increased protein expression in the lysate of neutrophils from the normal subject compared with the lysate from neutrophils of patient I.2.2; similarly, red spots indicate increased protein expression in the lysate of neutrophils from patient I.2.2. Convergence of spots resulting in yellow indicated equivalent protein expression in the lysates of neutrophils from both subjects. The spots that were differentially expressed are indicated by circles and identified by numbers. The identity of these differentially expressed spots was excised and determined by mass spectrometry. The inset is an enlargement of the region where Aip1 migrated. (B) Same analysis for normal compared with patient I.2.3. (C) Relative changes in the mean (normal vs patient) expression of proteins in neutrophil lysates. The number in each bar refers to the specific spot on the 2D gel. Note that both cofilin (*) and AIP1 (arrows) are represented in multiple spots. (D) Protein expression in neutrophil lysates from 3 normal subjects, patients I.2.2 and I.2.3, and their unaffected heterozygous sibling, I.2.1, and patient III.2.2 of family III. The immunoblots were probed with antibodies to Aip1, cofilin, phospho-cofilin Ser(3), and actin as indicated.
Cofilin activity is regulated by LIM kinase-mediated phosphorylation at Ser3, which disrupts the F-actin binding domain, resulting in decreased actin depolymerization. Using a phospho-specific Ser3-cofilin antibody, substantial phosphorylation of cofilin was observed in normal neutrophil lysates, whereas patient cofilin was largely unphosphorylated, suggesting increased cofilin-mediated depolymerization activity (Figure 5D, lanes 4 and 5). Although the differential expression of cofilin might have a profound impact on the neutrophil cytoskeleton, neutrophils from patient III.2.2 had normal phosphorylation of cofilin, suggesting that this was not the critical protein (Figure 5D, lane 7).
Three spots identified as Aip1 (Figure 5A-C) were differentially expressed in patient neutrophil lysates. DNA sequencing of the Aip1 encoding gene, WDR1, in family 1 identified the homozygous missense mutation c.76G>A, p.D26N (Polyphen-2 score = 1.00, sensitivity: 0.00, specificity: 1.00, “Probably damaging”) in patients I.2.2 and I.2.3. The unaffected parents, maternal uncle, and brother were heterozygous (supplemental Figure 4A). Aip1 was the first single protein recognized with 2 β-propellers, each consisting of 7 blades of a β-sheet formed by 4 antiparallel β-strands (denoted A, B, C, and D radially outward; Figure 1F).13 D26N lies in a highly conserved region of strand A of blade 1 of the N-terminal β-propeller (homology modeled human Aip1 based on Caenorhabditis elegans Aip1/UNC-78 (Protein Data Bank ID code 1NRO; 39% homology).14
Because of clinical phenotypic similarity, DNA was obtained from members of families II and III. In family II, the father of II.2.2 was heterozygous for a 3 nucleotide in-frame deletion c.19-21delAAG, p.delK7, whereas the mother was heterozygous for the missense mutation c.1270G>A, p.V424M (Polyphen-2 score = 0.903, sensitivity: 0.82, specificity: 0.94, “Possibly damaging”; supplemental Figure 4B). Both of these mutations fall within the C-terminal β-propeller, with the paternal mutation in the D strand of blade 14 and the maternal mutation in the B strand of blade 10 (Figure 1F). The patient’s sister, II.2.1, and one of her daughters, II.3.1, were heterozygous for c.1270G>A, p.V424M. Efforts to locate DNA on patient II.2.2, who had died in the 1970s, were not successful, but because of the similar phenotype, the severity of her disease, and the discovery of damaging mutations in WDR1 on both parental alleles, we surmise that II.2.2 was compound heterozygous for both parental mutations, resulting in clinical WDR1 deficiency.
Patient III.2.2 was compound heterozygous for WDR1 c.361G>A, p.G121R (Polyphen-2 score = 1.00, sensitivity: 0.00, specificity: 1.00, “Probably damaging”) and c.856C>G, p.L286V (Polyphen-2 score = 1.00, sensitivity: 0.00, specificity: 1.00, “Probably damaging”) (supplemental Figure 4C). His mother, III.1.1, was heterozygous only for c.856C>G, p.L286V, suggesting that the c.361G>A, p.G121R mutation was paternal, although we cannot formally exclude that it arose spontaneously. The father of III.2.2 was unavailable for testing. Both mutations were in the N-terminal β propeller. All 5 identified mutations fell within structurally invariant β-strand regions of the blades, predicting disruption of Aip1 function.
Discussion
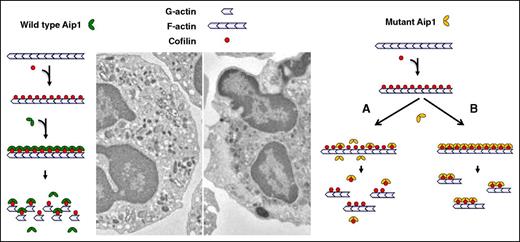
The actin cytoskeleton is required for the structural integrity of all cells. However, motile cells such as neutrophils require rapid and directional actin remodeling. Adherence to and spreading on endothelium and migration through the endothelium into tissue require morphologic changes that depend on the dynamic polymerization and depolymerization of cytoskeletal actin. These changes necessarily alter the movement of intracellular organelles as well as the overall fluidity of the cytosol (gel↔sol transformations) (Figure 6). Aip1 regulates actin disassembly and is expressed in most leukocytes, as well as cardiac myocytes, smooth muscle, and bronchial epithelium, but not in skeletal muscle. Cofilin severs F-actin and is involved in many cellular processes that require rapid actin turnover, such as lamellipod formation in migrating cells. Aip1 is thought to enhance the efficiency of cofilin, promoting the complete depolymerization of F-actin oligomers to monomeric actin. Although it has little severing activity on its own, Aip1 regulates cofilin by enhancing the depolymerization of F-actin to monomeric G-actin rather than to short oligomers.15 More recent findings suggest that Aip1 forms a ternary complex with cofilin and F-actin that destabilizes actin–actin monomer interactions, resulting in the rapid disassembly of actin filaments.16 Knockdown of Aip1 leads to abnormal and asymmetric accumulation of thicker actin filaments at the contractile ring in late telophase, disrupting normal cytokinesis and promoting multinucleate cells, a phenotype corrected by a constitutively active (Ser3 > Ala) cofilin.15
Proposed consequence of mutated Aip1. Neutrophil migration and spreading requires dynamic polymerization/depolymerization of the cytoskeletal actin, altering the fluidity of the cytosol (gel↔sol transformations) and promoting changes in cellular morphology. Aip1 is thought to bind to cofilin and enhance its actin depolymerizing activity. Mutations affecting Aip1 either disrupt its interactions with cofilin (failure to enhance its activity), promoting the accumulation of F-actin and disrupting the cytoskeletal organization of the cytosol.
Proposed consequence of mutated Aip1. Neutrophil migration and spreading requires dynamic polymerization/depolymerization of the cytoskeletal actin, altering the fluidity of the cytosol (gel↔sol transformations) and promoting changes in cellular morphology. Aip1 is thought to bind to cofilin and enhance its actin depolymerizing activity. Mutations affecting Aip1 either disrupt its interactions with cofilin (failure to enhance its activity), promoting the accumulation of F-actin and disrupting the cytoskeletal organization of the cytosol.
Aip1 is a dual β-propeller that consists of 11 WD repeats, a motif that generally consists of ∼40 amino acids bracketed by the amino acid pairs Gly-His on the amino end and Trp-Asp (WD) on the carboxyl end. Each propeller has 7 blades, and each blade consists of a small antiparallel β-sheet with 4 antiparallel β-strands. Most interspecies structural divergence in Aip1 resides in the loops connecting the β-strands, and not in the β-strands themselves, suggesting that those regions are less tolerant of variation. Each WD repeat comprises a single propeller blade; the remaining 3 blades do not contain WD repeat domains but still generate similar structural elements. Based on the crystal structure of yeast Aip1,13 there are dense patches of conserved residues on the surface of the N-terminal propeller and at the interface of the 2 propellers. Because the amino terminus forms the D strand of blade 7 of the C-terminal β-propeller, the polypeptide chain traverses the interface twice (amino acids 10-20 and 337-341), conferring rigidity to the hinge region, resulting in an open clamshell shape (angle 115°; Figure 1F).
Complete Aip1 deficiency is lethal during embryogenesis.17 However, the redears (rd) mouse has a single base mutation forming a cryptic splice site that results in a 6-base in-frame deletion and a loss of amino acids 346-347 in β-strand C of blade 8 of the C-terminal β-propeller. This mutation reduces Aip1 expression by 70% to 80% and manifests as macrothrombocytopenia and neutrophilia with inflammatory lesions of the ears, feet, and tail.17 Platelets and megakaryocytes are abnormal with large cytosolic regions devoid of granules, organelles, and membranes. The inflamed lesions are neutrophilic with necrosis and have been characterized as autoinflammatory. Redears neutrophils had impaired chemotaxis in vitro, elevated F-actin, and decreased depolymerization of F-actin in response to the chemokine, Mip-2. It remains unclear whether the redears lesions were autoinflammatory or due to infections with resident skin flora. Persistence of these lesions even on a T cell–deficient background does not exclude a critical role for neutrophil function. Recently, Kim et al suggested that autoinflammatory disease in the redears mouse was mediated by increased levels of plasma interleukin-18 (IL-18) thought to be produced by inflammatory monocytes.18 However, that physiology may not directly apply to Pts I.2.2 and I.2.3 because neither patient exhibited persistently elevated levels of IL-18, nor did lipopolysaccharide stimulation of patient PBMCs show exaggerated IL-18 production (data not shown). Moreover, the redears mouse exhibited neutrophilia, whereas the patients described here exhibited mild neutropenia.
We identified 5 disease alleles in 3 families leading to an overall similar clinical and laboratory phenotype: abnormal neutrophil morphology and function, mild neutropenia, recurrent bacterial infections, and, in some case, severe stomatitis, severe varicella zoster virus infection, and premature death. We saw no evidence of a heterozygous phenotype, confirming this as a true recessive disease. The mutation in family I, D26N, was easy to demonstrate in the probands. We were forced to infer the mutations in patient II.2.2, as we had no patient DNA for sequencing. However, the identification of distinct deleterious heterozygous mutations in her parents (mother, V424M; father, delK7) and V424M in a sibling and niece strongly suggest that mutated WDR1 was in fact the gene responsible for her illness and demise. In family III, we identified compound heterozygous mutations L286V and G121R. All mutations occurred at highly conserved amino acids located within invariant antiparallel β-strands that constitute the blades of the β-propeller of Aip1.
Mutated Aip1 protein levels in patient neutrophil lysates were comparable to Aip1 protein levels in normal neutrophil lysates (∼60-70% of normal and similar to an unaffected heterozygous sibling; Figure 5D, lane 6), as were the levels of cofilin. However, in family I, Ser3 phospho-cofilin was markedly reduced in the 2D gels. Phosphorylation of cofilin at Ser3 downregulates its actin-severing activity by disrupting the F-actin binding site. Therefore, cofilin in patient cells was constitutively active compared with cofilin in normal neutrophils However, this was not a consistent finding among patients with mutations in WDR1 (Figure 5D, lane 7). Despite a dramatic reduction in phosphorylation of Ser3 in these patients (with an expected increase in actin severing activity), neutrophils from both patients exhibited a two- to threefold increase in F-actin in their neutrophils. This underscores a previously unknown and critical role for Aip1 in actin disassembly. The abnormal Aip1 encountered in this disease appears unable to regulate cofilin activity normally, resulting in accumulation of perhaps shorter F-actin oligomers while concurrently expressing highly active actin-severing activity (Figure 5D). Efforts to rescue defective Aip1 in patient fibroblasts proved unsuccessful. Only limited additional expression of Aip1 was tolerated in normal fibroblasts; overexpression of Aip1 reduced cellular viability. We hypothesize that patient fibroblasts could not attain sufficient wild-type Aip1 expression to overcome the mutant protein expression without reducing cellular viability.
These mutations in WDR1 clearly separate the critical roles of Aip1 in neutrophils (and perhaps other leukocytes) from its roles in other cells. Overall growth and development of these patients was normal, suggesting that the function of Aip1 affected is most important for the rapid remodeling of the cytoskeleton that is intrinsic to leukocyte function, but apparently less critical for other cellular activities. This novel autosomal recessive immunodeficiency identifies a previously unappreciated role for Aip1 in cell biology, neutrophil function, and host defense.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health and has been funded in part with federal funds from National Cancer Institute, National Institutes of Health, contract HHSN261200800001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Authorship
Contribution: D.L.F., K.L., D.L.P., D.R., L.M., R.C., V.M., and A.K. performed the majority of the functional assays used to define the neutrophil defect; U.C., C.S., A.P.H., S.J.L., D.C., E.M., and J.S.O. designed and performed additional studies designed to further characterize the genetic defect; P.J.S. was responsible for the molecular modeling of Aip1; G.U., A.F.F., K.N.O., M.A.-A., V.L.A., E.K., H.L.M., J.I.G., and S.M.H. were responsible for the primary care of the patients; D.B.K. was responsible for the initial draft of the manuscript and analysis of the data; and D.B.K., J.I.G., K.A.Z., H.L.M., and S.M.H. were responsible for critical reading of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven M. Holland, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, NIH, Building 10/11N248 MSC 1881, Bethesda, MD 20892-1881; e-mail: smh@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal