Key Points
Mimicking 3D BM stiffness improves MK maturation with in situ–like morphology and higher ploidy and proplatelet formation.
The myosin IIA and MKL1 pathways contribute to 3D hydrogel medium-mediated increased proplatelet formation.
Abstract
Megakaryocyte (MK) differentiation occurs within the bone marrow (BM), a complex 3-dimensional (3D) environment of low stiffness exerting local external constraints. To evaluate the influence of the 3D mechanical constraints that MKs may encounter in vivo, we differentiated mouse BM progenitors in methylcellulose (MC) hydrogels tuned to mimic BM stiffness. We found that MKs grown in a medium of 30- to 60-Pa stiffness more closely resembled those in the BM in terms of demarcation membrane system (DMS) morphological aspect and exhibited higher ploidy levels, as compared with MKs in liquid culture. Following resuspension in a liquid medium, MC-grown MKs displayed twice as much proplatelet formation as cells grown in liquid culture. Thus, the MC gel, by mimicking external constraints, appeared to positively influence MK differentiation. To determine whether MKs adapt to extracellular stiffness through mechanotransduction involving actomyosin-based modulation of the intracellular tension, myosin-deficient (Myh9−/−) progenitors were grown in MC gels. Absence of myosin resulted in abnormal cell deformation and strongly decreased proplatelet formation, similarly to features observed for Myh9−/− MKs differentiated in situ but not in vitro. Moreover, megakaryoblastic leukemia 1 (MKL1), a well-known actor in mechanotransduction, was found to be preferentially relocated within the nucleus of MC-differentiated MKs, whereas its inhibition prevented MC-mediated increased proplatelet formation. Altogether, these data show that a 3D medium mimicking BM stiffness contributes, through the myosin IIA and MKL1 pathways, to a more favorable in vitro environment for MK differentiation, which ultimately translates into increased proplatelet production.
Introduction
Bone marrow (BM) is a highly cellular and dynamic tissue composed mostly of hematopoietic cells at all stages of differentiation, which are in constant renewal and undergo migration from the stem cell niche to the vascular sinusoids. Some stromal cells are also present, surrounded by a loose network of extracellular matrices composed essentially of fibrillar proteins and glycosaminoglycans, which form a physically elastic network to which cells can adhere.1,2 Within this complex environment, megakaryocytes (MKs) interact with other cells and protein matrices in a 3-dimensional (3D) configuration. They are exposed to a soft elastic medium with a Young’s modulus estimated to lie between 15 and 300 Pa,3,4 well below that of muscle (≈10 kPa) or even brain (≤1 kPa).5-7 The relationship between substrate stiffness and cell differentiation has been studied in a number of cellular systems, and it is now thought that substrate elasticity, by converting mechanical inputs into intracellular signals, controls the development of stem cell fates, including hematopoietic stem cells (HSCs), during their proliferation and differentiation.3,8
MKs are formed from HSCs through a complex and well-orchestrated process of differentiation and maturation. During this process, progenitors undergo several rounds of endomitoses, acquiring a large polyploid nucleus.9 In the late stages, the cytoplasm considerably enlarges simultaneously with the development of a broad network of intracellular membranes called demarcation membrane system (DMS).10 These membranes organize to form an intertwined tubular network that further flattens to form membrane sheets.11 In the more mature stage III MKs observed in situ,12 DMS membranes occupy most of the cytoplasm with the exception of perinuclear and peripheral zones. On transmission electron microscopy (TEM) sections, they appear to be closely apposed and delineate homogeneous cytoplasmic areas, initially called “platelet territories” because they were thought to be the preformed future platelets.13-16 It is now considered that platelets are not preformed within MKs, but that DMS membranes serve as a reservoir for the membrane of the future platelets. Platelets are produced at the end of proplatelets, long cytoplasmic protrusions of the MK, projected through the endothelial sinusoid barrier.17,18 It is thought that the proplatelets then further remodel and release platelets into the blood circulation. However, recent findings have also revealed an alternative process without extension of long protrusions19,20
We previously reported that in absence of myosin IIA, MKs paradoxically displayed either decreased or increased proplatelet formation, depending on whether they were differentiated in situ or in vitro, respectively.21,22 This finding suggested that the in situ environment where MKs differentiate contributes in some way to the final platelet production process through modulation of the actomyosin cytoskeleton, possibly through adaptation of cells to the environmental physical constraints. We thus hypothesized that mimicking in vitro these mechanical constraints, which the progenitors encounter in situ, could improve in vitro MK differentiation and maturation, with consequences for platelet formation. To mimic both the 3D aspect and the physical constraints, we used a 3D culture system based on a methylcellulose (MC) hydrogel known to be compatible with the development of colony-forming unit–MK.23 We show here that in an MC gel with a defined stiffness in the range of that of the BM, MKs reach a higher ploidy than in liquid 2-dimensional culture and exhibit a DMS that more closely resembles that observed in vivo. Furthermore, MKs grown in MC gel have an increased capacity to form proplatelets. We propose that the external mechanical constraints activate the cellular mechanotransduction machinery and show that both myosin IIA and megakaryoblastic leukemia 1 (MKL1) contribute to this MC-mediated increased proplatelet formation.
Materials and methods
Materials
Materials are described in the supplemental Methods section, available on the Blood Web site.
Mice
All mice had a C57BL/6 background and were 2 to 4 months old. Wild-type (WT) mice were purchased from Charles River (L’Arbresle, France).The floxed Myh9 strain was crossed with PF4-Cre mice24 to obtain animals with deletion of the Myh9 exon 1 (Myh9−/− mice) in the megakaryocytic lineage, as described previously.25
Culture of mouse BM Lin− progenitor cells
Mouse BM Lin− cells (referred to as Lin− cells) were cultured as described previously26 (see supplemental Methods). In some experiments, E13.5-14.5 fetal Lin− cells were also assayed. For culture in MC gels, cells were encapsulated in the gel at room temperature. In some cultures, the MKL1 inhibitor CCG-1423 (10 µM) was added to the medium at the time of encapsulation and after resuspension of the cells on day 3.
In vitro proplatelet formation
Cells were recovered from the gels after 3 days of culture by dilution of the MC gel and resuspension in complete liquid medium, and the proportion of MK forming proplatelets was determined at various time points (see supplemental Methods).
Rheological measurements
The mechanical properties of MC hydrogels were deduced by measurement of the complex shear modulus using a Haake, Mars III stress-controlled rheometer in the oscillatory mode (see supplemental Methods).
TEM
Cells were fixed in 2.5% glutaraldehyde directly in the culture wells to prevent morphological changes due to cell resuspension and processed for TEM (see supplemental Methods). To directly observe in situ MKs, BMs were flushed either directly in 2.5% glutaraldehyde or in collagenase (3 mg/mL) for isolation of the cells and resuspension before fixation (see supplemental Methods).
Confocal microscopy
On day 3 of culture, cells were fixed in 4% paraformaldehyde directly in gels. Following gel dilution and centrifugation, cells were cytospun and processed for immunolabeling. Plateletlike fragments were recovered and processed for analysis (see supplemental Methods). All confocal images were acquired in the equatorial plane. Fluorescence was quantified using ImageJ software as described by Burgess et al.27 The circularity or shape factor was analyzed using ImageJ software, according to Khatau et al.28
Flow cytometry
The ploidy level was determined using propidium iodide22 and analyzed using a BD LSRFortessa X20 flow cytometer and BD FacsDiva software (Becton Dickinson, Le Pont-de-Claix, France).
Results
Normal MK maturation requires the BM environment
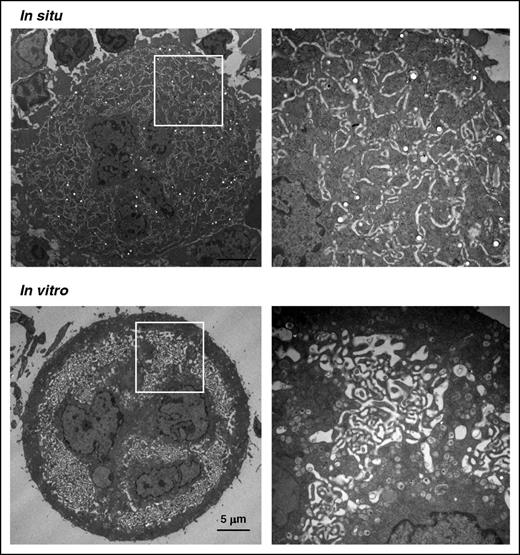
Differentiation of HSCs in vitro has become an essential tool in the study of MK biology since the identification of thrombopoietin. However, when examining the ultrastructure of mouse MKs following Lin− cell differentiation in liquid culture, major differences can be observed as compared with cells differentiated in their native environment (Figure 1). The most noticeable difference involves the DMS. In most cells, the membranes have the appearance of small round, oval, or elongated vesicles without delimitation of cytoplasmic territories (Figure 1 lower right). Whether this is due to abnormal or incomplete maturation is unclear, but 1 likely explanation is that it results from the absence of the BM environment. One component of this environment is the presence of surrounding local external mechanical forces. We therefore reasoned that growing progenitors in a 3D medium presenting a stiffness close to that of the BM could have an impact on MK maturation.
Different ultrastructures of mature MKs differentiated in situ or in vitro. Electron microscopy images of WT murine MKs differentiated in the BM (in situ, upper panel) or in liquid culture in vitro (lower panel). The images of whole cells (left) and close-up views (right) are representative of at least 4 different mouse BM samples (in situ) and 10 different liquid cultures.
Different ultrastructures of mature MKs differentiated in situ or in vitro. Electron microscopy images of WT murine MKs differentiated in the BM (in situ, upper panel) or in liquid culture in vitro (lower panel). The images of whole cells (left) and close-up views (right) are representative of at least 4 different mouse BM samples (in situ) and 10 different liquid cultures.
Mechanical characterization of MC hydrogels
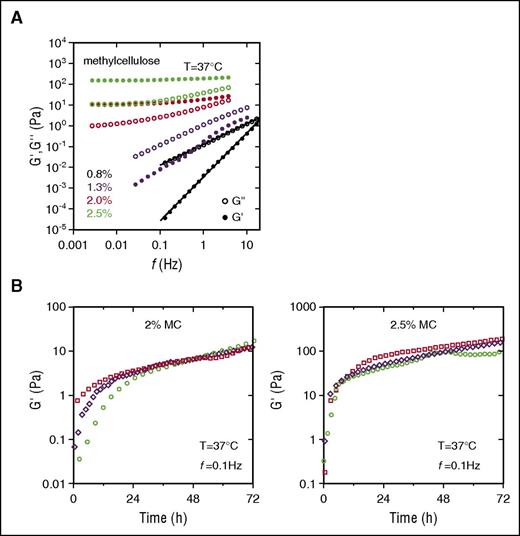
MC was chosen to encapsulate Lin− cells because it has long been used in clonogenic assays and for this reason is known to be compatible with hematopoietic progenitor growth. The results of shear measurements performed on aqueous MC solutions at various concentrations after annealing for 72 hours at 37°C are shown in Figure 2A. At a concentration of 0.8%, the elastic part G′ and the viscous part G″ of the shear modulus exhibit the low-frequency terminal response characteristic of a liquid state given by G′ ∼ f2 and G″ ∼ f, where f is the frequency. At a concentration of 1.3%, classically used for clonogenic assays, the behavior of the G′ and G″ parts of the modulus corresponds to the response of a solution with aggregates. In contrast, at concentrations of 2% and 2.5%, the elastic contribution dominates the viscous contribution and displays an elastic plateau at low frequency, characteristic of the gelled state. As expected for MC hydrogels, extending the annealing time increases the stiffness of the network, as shown by the evolution of the G′ values.
Viscoelastic properties of MC. (A) Frequency sweep at 37°C for MC at different concentrations (0.8% to 2.5%). Curves are representative of 3 different samples for each concentration. G′ (filled symbols) and G″ (unfilled symbols) represent the elastic and viscous parts, respectively, of the complex shear modulus and are both expressed in Pascals. (B) Evolution of the shear modulus over 3 days in 2% MC (left) and 2.5% MC (right) at a constant oscillatory frequency.
Viscoelastic properties of MC. (A) Frequency sweep at 37°C for MC at different concentrations (0.8% to 2.5%). Curves are representative of 3 different samples for each concentration. G′ (filled symbols) and G″ (unfilled symbols) represent the elastic and viscous parts, respectively, of the complex shear modulus and are both expressed in Pascals. (B) Evolution of the shear modulus over 3 days in 2% MC (left) and 2.5% MC (right) at a constant oscillatory frequency.
At 2% MC, G′ lay in the range 10 to 20 Pa after an annealing time of 72 hours (Figure 2B). An increase in MC concentration of only 0.5% led to a strong increase in the stiffness of the hydrogel (10-fold, G′ of 100-200 Pa). Assuming a Poisson’s ratio ν of 0.5, these G′ values correspond to Young’s modulus values in the ranges 30 to 60 and 300 to 600 Pa at 2% and 2.5% MC, respectively, and are of the same order as those reported for BM (Emarrow = 15-300 Pa).3,4 Thus, hydrogels with MC concentration of 2% or 2.5% were further tested as a 3D medium for MK differentiation.
In situ–like morphology of the DMS is dependent on medium stiffness
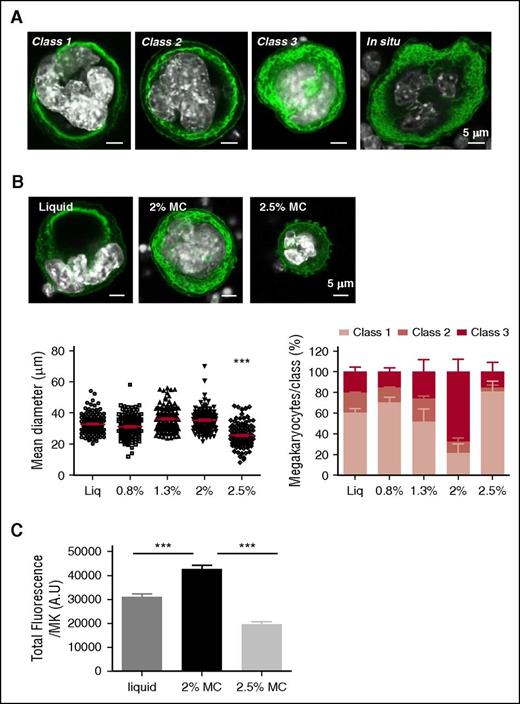
On day 0, Lin− progenitors were either seeded in liquid medium or encapsulated in 2% or 2.5% MC. After 3 to 5 days, a similar viability and number of MKs was observed in liquid cultures and 2% MC, but the number of MKs was decreased 4-fold in 2.5% MC (supplemental Figure 1A-B). DMS amount was evaluated using anti-GPIbβ immunolabeling and fluorescence microscopy.11 MKs with distinct amount and morphology of the DMS were classified according to the presence of a mainly peripheral DMS that does not delineate cytoplasmic territories (class 1), a cytoplasm filled with a DMS presenting cytoplasmic territories resembling that of in situ mature type III MKs (class 3) and class 2 cells, intermediate between classes 1 and 3 (Figure 3A). Increasing the MC concentration from 0.8% to 2% did not significantly modify the size of MKs (Figure 3B lower left). However, it shifted the development of the DMS toward the class 2 to 3, thus promoting an in situ–like morphology (Figure 3B lower right). In addition, the amount of DMS estimated from the GPIbβ immunofluorescence intensity was significantly increased in 2% MC gels as compared with liquid cultures (Figure 3C). To examine whether the 3D confinement was absolutely required in addition to the stiffness of the substrate, we grew cells on top of 2% MC gels. Under these conditions, MKs adopted the morphology of liquid-grown cells, with a majority of class 1 MKs (supplemental Figure 2). Thus, the 3D environment, in addition to an MC concentration of 2%, appeared to confer optimal stiffness, and increasing the rigidity 10-fold (2.5% MC) significantly decreased MK size (Figure 3B) and DMS development.
MC-cultured WT MKs display in situ–like DMS development and morphology. (A) Confocal microscopy images of MKs from either 3-day in vitro cultures or in situ BM section, showing the various morphologies of the DMS. The plasma membrane and DMS are labeled with an anti-GPIbβ antibody (green) and the nucleus with 4′,6-diamidino-2-phenylindole (DAPI; white). Images are representative of at least 3 different experiments. Scale bar = 5 µm. (B) Impact of the MC medium on MK morphology following 3-day culture. In the upper panel, the DMS appears in green and the nucleus appears in white as in (A). Scale bar = 5 µm. In the lower panel, the mean diameter of MKs (left) and their proportion in each class (right) are shown for liquid and MC hydrogel (0.8, 1.3, 2 and 2.5%) cultures. Results are expressed as the mean ± SEM in 3 independent cultures, with a total of at least 100 MKs examined. ***P < .0001 using 1-way analysis of variance (ANOVA) with Bartlett’s test comparing the 2.5% MC condition to all other conditions. (C) Bar graphs represent the total cell fluorescence for GPIbβ staining in liquid cultures and 2% or 2.5% MC hydrogels. Data are representative of 3 different cultures for each condition, with a total number of 137, 134, and 120 MKs analyzed for liquid cultures and 2% and 2.5% MC gels, respectively. Results are expressed as the mean ± SEM: ***P < .0001 using a 1-way ANOVA analysis with a Newman-Keuls posttest.
MC-cultured WT MKs display in situ–like DMS development and morphology. (A) Confocal microscopy images of MKs from either 3-day in vitro cultures or in situ BM section, showing the various morphologies of the DMS. The plasma membrane and DMS are labeled with an anti-GPIbβ antibody (green) and the nucleus with 4′,6-diamidino-2-phenylindole (DAPI; white). Images are representative of at least 3 different experiments. Scale bar = 5 µm. (B) Impact of the MC medium on MK morphology following 3-day culture. In the upper panel, the DMS appears in green and the nucleus appears in white as in (A). Scale bar = 5 µm. In the lower panel, the mean diameter of MKs (left) and their proportion in each class (right) are shown for liquid and MC hydrogel (0.8, 1.3, 2 and 2.5%) cultures. Results are expressed as the mean ± SEM in 3 independent cultures, with a total of at least 100 MKs examined. ***P < .0001 using 1-way analysis of variance (ANOVA) with Bartlett’s test comparing the 2.5% MC condition to all other conditions. (C) Bar graphs represent the total cell fluorescence for GPIbβ staining in liquid cultures and 2% or 2.5% MC hydrogels. Data are representative of 3 different cultures for each condition, with a total number of 137, 134, and 120 MKs analyzed for liquid cultures and 2% and 2.5% MC gels, respectively. Results are expressed as the mean ± SEM: ***P < .0001 using a 1-way ANOVA analysis with a Newman-Keuls posttest.
A more precise insight into DMS aspect was obtained by TEM analyses. Contrary to that of cells in liquid culture, the DMS of class 3 MKs grown in 2% MC, delimiting cytoplasmic territories, had membranes that were closely apposed, as in MKs in situ (Figure 4A). In contrast, cells differentiated in 2.5% MC appeared to be mostly immature and contained very small amounts of DMS, in accordance with their smaller size (supplemental Figure 3). To determine whether our observations could be generalized to embryonic progenitors, we differentiated Lin− cells from fetal liver. Similarly to adult progenitors, MKs from embryonic progenitors differentiated in an MC gel exhibited DMS membranes closely apposed that also may delineate cytoplasmic territories, again contrary to MKs grown in liquid medium (supplemental Figure S4A). Thus, growing cells in a soft 3D MC hydrogel with a low stiffness similar to that of BM favored the appearance of a nativelike morphology of the DMS of MKs from both adult or embryonic origin.
DMS morphology depends on the stiffness of the medium. (A) Electron microscopy images of stage III BM MKs (in situ) or MKs differentiated for 3 days in liquid or 2% MC cultures (class 3). Scale bar = 2 µm. Images are representative of at least 4 mice (in situ) or 10 cultures. (B) Left, electron microscopy image of an MK differentiated for 3 days in 2% MC and resuspended in a liquid medium for 2 hours before fixation (representative of 3 independent experiments with >90 MKs examined). Right, close-up view of the cytoplasm of the MK. (C) Left, electron microscopy image of an MK from BM after dissociation and resuspension for 2 hours in a liquid medium (representative of 3 independent experiments with >90 MKs examined). Right, close-up view of the cytoplasm of the MK.
DMS morphology depends on the stiffness of the medium. (A) Electron microscopy images of stage III BM MKs (in situ) or MKs differentiated for 3 days in liquid or 2% MC cultures (class 3). Scale bar = 2 µm. Images are representative of at least 4 mice (in situ) or 10 cultures. (B) Left, electron microscopy image of an MK differentiated for 3 days in 2% MC and resuspended in a liquid medium for 2 hours before fixation (representative of 3 independent experiments with >90 MKs examined). Right, close-up view of the cytoplasm of the MK. (C) Left, electron microscopy image of an MK from BM after dissociation and resuspension for 2 hours in a liquid medium (representative of 3 independent experiments with >90 MKs examined). Right, close-up view of the cytoplasm of the MK.
To determine how MKs behave when local external constraints are relieved, cells grown in MC gel were resuspended in liquid medium for 2 hours before fixation for TEM. Interestingly, we found that the DMS membranes were remodeled and acquired a structure resembling that observed when MKs were continuously grown in liquid medium (Figure 4B, compare with Figures 1 and 4A). In particular, the DMS was mostly forming vacuoles or vesicles; its flattened aspect had disappeared, and it no longer delimited cytoplasmic territories. These surprising observations suggested that the DMS is highly dynamic and can rapidly remodel.
Interestingly, this was also the case when native MKs directly harvested from BM were resuspended in liquid medium for 2 hours. Remarkably, these cells lost the DMS morphology typical of in situ stage III MKs (see Figure 1 upper panel) and the DMS similarly adopted a vacuolated and noncontinuous aspect (Figure 4C). Conversely, we asked whether MKs grown in liquid medium would adopt an in situ–like morphology upon inclusion in MC gel. After 3 days of liquid culture followed by cell encapsulation for 4 or 24 hours, we observed a trend toward increased class 2 and 3 morphologies compared with liquid culture (supplemental Figure 5).
Altogether, these results indicate that the morphology of the DMS depends on the mechanical constraints of the environmental medium.
Culture in MC hydrogels improves MK differentiation and proplatelet formation
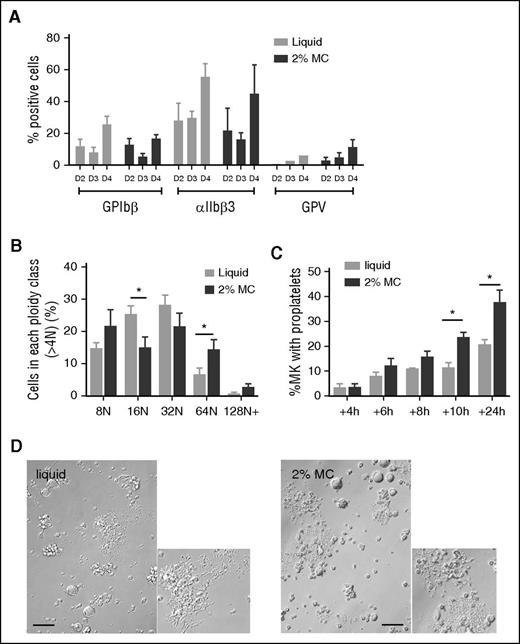
We next examined whether the progenitor culture in MC gel led to an overall increase in MK differentiation. The integrin αIIbβ3, GPIbβ, and GPV markers increased upon differentiation but had a similar expression pattern between liquid and MC cultures (Figure 5A). The ploidy of MKs >4 N was overall increased in cells cultured in MC gels, with a significant lower amount of 16 N and a higher amount of 64 and 128 N (Figure 5B).
MC culture improves MK ploidy and proplatelet formation. (A) Expression of MK markers (αIIbβ3, GPIbβ, and GPV) on Lin− cells after 2, 3, or 4 days in liquid or 2% MC culture (% of total viable cells). Bar graphs represent 3 different flow cytometric analyses of 3 independent cultures, and results are expressed as the mean ± SEM. No significant difference observed between both culture conditions at each time point (2-way ANOVA). (B) Flow cytometric analysis of the percentage of MKs in each ploidy class at day 4 of differentiation, considering only cells of ploidy > 4 N. Bar graphs are representative of 6 independent experiments. *P < .05 using Student t test. Mean ploidy ± SEM: 33.18 ± 3.55 N for MC cultures vs 25.58 ± 2.20 N for liquid cultures (n = 6, P = .09). (C) Bar graphs represent the percentages of cells extending proplatelets in liquid and 2% MC cultures after 4, 6, 8, 10, or 24 hours following resuspension in liquid medium. A total of at least 300 MKs were examined in each kinetic. Results are the mean ± SEM in 4 independent experiments; *P < .05 using Student t test. (D) Images of MKs bearing proplatelets after 3 days in liquid or 2% MC medium followed by 1 day resuspension in liquid medium.
MC culture improves MK ploidy and proplatelet formation. (A) Expression of MK markers (αIIbβ3, GPIbβ, and GPV) on Lin− cells after 2, 3, or 4 days in liquid or 2% MC culture (% of total viable cells). Bar graphs represent 3 different flow cytometric analyses of 3 independent cultures, and results are expressed as the mean ± SEM. No significant difference observed between both culture conditions at each time point (2-way ANOVA). (B) Flow cytometric analysis of the percentage of MKs in each ploidy class at day 4 of differentiation, considering only cells of ploidy > 4 N. Bar graphs are representative of 6 independent experiments. *P < .05 using Student t test. Mean ploidy ± SEM: 33.18 ± 3.55 N for MC cultures vs 25.58 ± 2.20 N for liquid cultures (n = 6, P = .09). (C) Bar graphs represent the percentages of cells extending proplatelets in liquid and 2% MC cultures after 4, 6, 8, 10, or 24 hours following resuspension in liquid medium. A total of at least 300 MKs were examined in each kinetic. Results are the mean ± SEM in 4 independent experiments; *P < .05 using Student t test. (D) Images of MKs bearing proplatelets after 3 days in liquid or 2% MC medium followed by 1 day resuspension in liquid medium.
We then looked at the capacity of MC-grown MKs to form proplatelets. As they do not extend proplatelets within the gel, this property was analyzed after resuspension of the cells in liquid medium. Proplatelet formation began 4 hours following resuspension and gradually increased (Figure 5C). MC-grown MKs extended more proplatelets than liquid-grown MKs as early as 10 hours and reached 42.2% after 24 hours compared with 22.6% for liquid-grown MKs (P < .05) (Figure 5C). A similar difference was also observed when fetal liver Lin− cells were similarly processed (33.6 vs 63.5% MKs forming proplatelet for liquid vs MC culture after 24 hour resuspension) (supplemental Figure 4B).
The proplatelets, observed by light microscopy, adopted a similar morphology whether cells had been previously differentiated in liquid or MC medium, with thin shafts, branchings, and proplatelet buds at their extremities (Figure 5D). Following pipetting to release plateletlike elements, we observed the presence of barbell platelets as well as discoid platelets exhibiting a marginal band, and able to become activated and fully spread upon adhesion to fibrinogen (supplemental Figure 6). Overall, these data indicate that growing hematopoietic progenitors in an MC gel of low stiffness modifies the way MKs differentiate with positive consequences in proplatelet formation.
The MC-mediated increase in proplatelet formation depends on myosin IIA
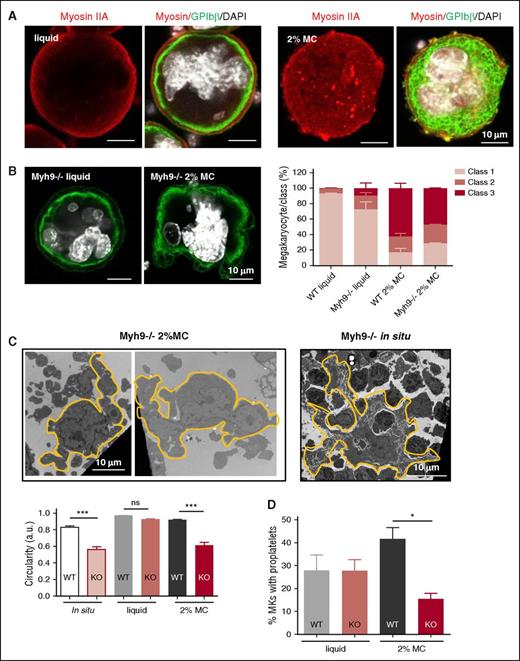
Cells adapt to extracellular stiffness by regulating their intracellular tension in response to mechanotransduction signals, possibly through modulation of actomyosin contractility. To determine whether myosin was involved, we first looked at myosin IIA distribution within cells. As shown in Figure 6A, myosin appeared mostly localized at the cell cortex in all the MKs grown in liquid medium. By contrast, the cortical myosin IIA distribution became discontinuous in MC-grown MKs, and some myosin patches appeared within the cytoplasm. In addition, quantification of myosin labeling showed a slight but significant increase when MKs were grown in MC gel (mean ± standard error of the mean [SEM]: 54 212 ± 2505 a.u. for MKs differentiated in MC vs 46 595 ± 2271 for MKs in liquid, P < 0.05). These data clearly pointed to a role of myosin IIA in cell adaptation to the extracellular medium, which we next investigated using myosin-deficient progenitors (Myh9−/−). The absence of myosin had no significant impact on MC-mediated DMS aspect as observed by confocal microscopy, and the classification of DMS morphology was similar in Myh9−/− and WT cultures (Figure 6B). However, we found that Myh9−/− MKs grown in MC gel adopted an abnormal, noncircular morphology. TEM analyses after glutaraldehyde fixation to better preserve the cell shape showed cells having extensions or constricted areas. This morphology was never observed in the WT and was reminiscent of the “leaky” MK morphology present in situ in Myh9−/− BM (Figure 6C).22 The cell shape index was calculated as a measure of circularity and was found to be decreased by 33% in Myh9−/− MKs grown in an MC hydrogel, reproducing the in situ picture (Figure 6C). By contrast, MKs grown in a liquid medium displayed almost perfect circularity whatever the genotype (Figure 6C and supplemental Figure 7). Interestingly, Myh9−/− cells differentiated in liquid medium were also ultrastructurally very close to their WT counterparts (supplemental Figure 7). Altogether, these findings point to the involvement of myosin IIA in the mechanism of MK adaptation to medium stiffness.
Differential involvement of myosin IIA in MC-mediated MK culture. (A) Myosin IIA immunolabeling (red) along with GPIbβ-positive membranes (green) and nucleus (DAPI, white). (B) Left, confocal microscopy images of Myh9−/− MKs in liquid or 2% MC cultures. Right, bar graphs represent the proportions of Myh9−/− cells in the different MK classes in liquid and 2% MC cultures, as compared with WT cells. Results are expressed as the mean ± SEM and are from 3 independent experiments, with a total of 86-93 MKs examined per condition. (C) Upper panel, electron microscopy images of Myh9−/− MKs in 2% MC cultures (left), as compared with in situ MKs (right). Images are representative of at least 6 different cultures and 4 different Myh9−/− BMs. Lower panel, bar graphs of the circularity of WT and Myh9−/− MKs in situ and in liquid and 2% MC media. Results are the mean ± SEM in 3 independent experiments, with a total of 37-59 cells examined per condition. ***P < .0001 using an ANOVA analysis and a Newman-Keuls posttest; ns, not significant. (D) Bar graphs represent the percentages of MKs bearing proplatelets in Myh9−/− liquid and 2% MC cultures, as compared with WT cells. Results are the mean ± SEM in 3 independent experiments, with a total of 428-557 MKs examined per condition. *P < .05 using an ANOVA analysis and a Newman-Keuls posttest. KO, Myh9 knockout.
Differential involvement of myosin IIA in MC-mediated MK culture. (A) Myosin IIA immunolabeling (red) along with GPIbβ-positive membranes (green) and nucleus (DAPI, white). (B) Left, confocal microscopy images of Myh9−/− MKs in liquid or 2% MC cultures. Right, bar graphs represent the proportions of Myh9−/− cells in the different MK classes in liquid and 2% MC cultures, as compared with WT cells. Results are expressed as the mean ± SEM and are from 3 independent experiments, with a total of 86-93 MKs examined per condition. (C) Upper panel, electron microscopy images of Myh9−/− MKs in 2% MC cultures (left), as compared with in situ MKs (right). Images are representative of at least 6 different cultures and 4 different Myh9−/− BMs. Lower panel, bar graphs of the circularity of WT and Myh9−/− MKs in situ and in liquid and 2% MC media. Results are the mean ± SEM in 3 independent experiments, with a total of 37-59 cells examined per condition. ***P < .0001 using an ANOVA analysis and a Newman-Keuls posttest; ns, not significant. (D) Bar graphs represent the percentages of MKs bearing proplatelets in Myh9−/− liquid and 2% MC cultures, as compared with WT cells. Results are the mean ± SEM in 3 independent experiments, with a total of 428-557 MKs examined per condition. *P < .05 using an ANOVA analysis and a Newman-Keuls posttest. KO, Myh9 knockout.
Strikingly, we observed that the absence of myosin IIA abolished the increase in proplatelet formation observed in MKs grown in MC gels and even decreased proplatelet production, whereas it had no impact on cells in liquid culture (Figure 6D). These features are reminiscent of the previously described paradoxical phenotype of Myh9−/− mice.22,29 Therefore, these data show that myosin IIA appeared to be dispensable for proplatelet formation in liquid culture. By contrast, the 3D MC medium re-creates some in vivo conditions that allow the recovery of the in situ Myh9−/− impaired phenotype.
MC-mediated increased proplatelet formation is promoted by nuclear accumulation of MKL1
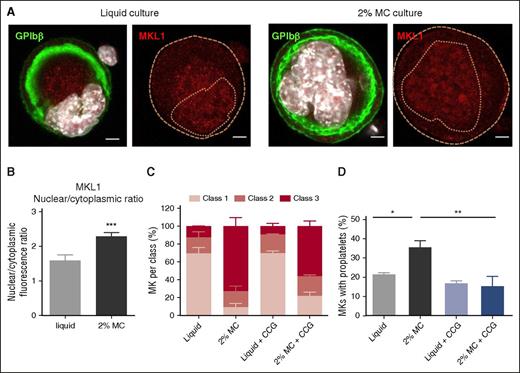
Among the effectors involved in mechanotransduction machinery, MKL1 appeared to be a likely candidate as a transducer of environmental stiffness30-32 and is likewise implicated in megakaryocytic differentiation.33-35 In response to an increase in cell rigidity and tension created by the surrounding substrate, MKL1 is known to shuttle from the cytoplasm to the nucleus, where it accumulates.33-36 MKL1 immunolabeling revealed an increased accumulation within the nucleus in MKs differentiated in an MC gel as compared with liquid culture (Figure 7A), with a 33% gain in the ratio of nuclear to cytoplasmic fluorescence (Figure 7B).
Proplatelet formation is promoted by nuclear accumulation of MKL1. (A) Confocal microscopy images of WT MKs after 3 days of culture in a liquid or 2% MC medium. The DMS and plasma membrane are visualized in green by immunolabeling with anti-GPIbβ; MKL1 immunolabeling is depicted in red, and nuclei appear in white (DAPI staining). The dotted lines delineate cell and nuclear margins. Images are representative of 3 independent experiments. (B) Bar graphs represent the MKL1 fluorescence in the cytoplasm and nucleus and the nuclear/cytoplasmic ratio, in MKs in liquid and 2% MC cultures. Results are the mean ± SEM in 3 cultures, with a total of 189-192 cells analyzed per condition. ***P < .0001 using Student t test. (C, D) Class distribution and proplatelet formation in MKs grown in a liquid or 2% MC medium, in the presence or absence of 10 µM CCG-1423. Results are the mean ± SEM in 3 independent cultures, with a total of 90-94 cells examined per condition (C), or in 4 independent cultures, with a total of 510-800 cells examined per condition (D). *P < .05, **P < .01 using ANOVA analysis and a Newman-Keuls posttest.
Proplatelet formation is promoted by nuclear accumulation of MKL1. (A) Confocal microscopy images of WT MKs after 3 days of culture in a liquid or 2% MC medium. The DMS and plasma membrane are visualized in green by immunolabeling with anti-GPIbβ; MKL1 immunolabeling is depicted in red, and nuclei appear in white (DAPI staining). The dotted lines delineate cell and nuclear margins. Images are representative of 3 independent experiments. (B) Bar graphs represent the MKL1 fluorescence in the cytoplasm and nucleus and the nuclear/cytoplasmic ratio, in MKs in liquid and 2% MC cultures. Results are the mean ± SEM in 3 cultures, with a total of 189-192 cells analyzed per condition. ***P < .0001 using Student t test. (C, D) Class distribution and proplatelet formation in MKs grown in a liquid or 2% MC medium, in the presence or absence of 10 µM CCG-1423. Results are the mean ± SEM in 3 independent cultures, with a total of 90-94 cells examined per condition (C), or in 4 independent cultures, with a total of 510-800 cells examined per condition (D). *P < .05, **P < .01 using ANOVA analysis and a Newman-Keuls posttest.
To examine the functional importance of this MKL1 nuclear translocation, MKs were differentiated in the presence of a specific small molecule inhibitor of MKL1, CCG-1423.35,37 CCG-1423 had only a minor impact on the class distribution according to DMS morphology (Figure 7C). However, although CCG-1423 had no impact on proplatelet formation in liquid cultures, suggesting that under our liquid culture conditions, MKL1 does not play a major role (Figure 7D), it totally abolished MC-mediated increased proplatelet production. This observation suggests that the MKL1 nuclear relocation occurring during MK differentiation in a 2% MC medium has a functional significance and contributes to the subsequent increase in proplatelet formation, as compared with MKs differentiated in a liquid medium.
Discussion
BM is a cohesive tissue forming a complex microenvironment consisting of cells and extracellular matrices organized in 3D.38 Because hematopoietic cells undergo constant renewal and migration to the blood vessels, contact and adhesion generating interactions and forces between adjacent cells and the matrix are unavoidable. In this work, we investigated simultaneously the impact of the 3D aspect and that of medium stiffness to mimic BM constraints. We showed that growing cells in a simple 3D hydrogel was sufficient to significantly improve MK differentiation in comparison with liquid cultures, as evidenced by a higher cell ploidy and modification of their ultrastructure to approach that of mature MKs in situ. Ultimately, differentiation in MC gel led to an increase in the number of MKs forming proplatelets.
A number of studies have already reported a role of substrate elasticity in enhancing hematopoietic progenitor/stem cell viability and expansion, which suggests that these cells can sense and react to the physical signals provided by the environment.3,39-41 Recently, Di Buduo et al found that a low stiffness supported higher proplatelet formation.42 However, these results were obtained after adhesion of MKs to silk having a stiffness in MPa range, far exceeding that of BM. A study by the group of Discher using a more physiological stiffness range showed that collagen I on soft gels promoted higher ploidy and proplatelets.43 In our study, the gel formulation was tuned to mimic the mechanical characteristics of BM, whose stiffness has been estimated to range from 15 to 300 Pa.3,4 Relatively small incremental changes in MC concentration (from 2% to 2.5%) increased the elastic modulus 10-fold. We found that the softer gel (2%, 30-60 Pa) was the optimal substrate for MK differentiation, DMS development, and proplatelet formation. The dramatic difference in MK culture between 2% and 2.5% MC would point to a role of mechanical forces, although we cannot totally rule out an impact of interaction with matrix proteins as our medium contains serum which can provide fibrin, von Willebrand factor, and fibronectin. In addition, the observations that the DMS morphology can switch from in situ–like to liquidlike morphology, or conversely, depending on the external medium, clearly argue for a component of the matrix stiffness. Our data suggest that differentiation/maturation is favored when MKs are in a softer environment, like those they may encounter around sinusoid vessels, unlike the stiffer environments encountered by cells closer to the endosteum. These observations are in agreement with Malara et al,44 who showed the presence in situ of a gradient of maturing MKs, based on their size, from the endosteal niche (supposedly more rigid) to vascular compartments (supposedly less rigid).
The mechanisms through which environmental elasticity promotes a higher amount of DMS with in situ–like morphology remain to be established. DMS formation is initiated by plasma membrane invagination and fueled by Golgi complex– and endoplasmic reticulum–mediated lipid transfer.10,11,45 How this membrane network is organized and its structure is maintained within the cytoplasm is still not clear. A role of F-actin meshwork, assembled through the WASP-WAVE pathway at its cytoplasmic face, has been proposed.45 Inactivation of genes related to actin dynamics during the maturation process leads to DMS abnormalities, as likewise freeing the filamin A–mediated membrane-to-cytoskeleton link.46-50 Moreover, ex vivo incubation of BM with cytochalasin D or latrunculin A results in strong vacuolization of the DMS (F.P. and C.L., unpublished data), again suggesting a key role of F-actin in maintenance of the membrane structure once it has developed. Spectrin, which forms a lattice underlying the DMS, also plays an important part as a cytoskeletal support for DMS development.48 It is possible that the external forces exerted by the MC gel have an impact on interactions between F-actin, spectrin, and the DMS membranes, which are rapidly modified upon cell resuspension. Interestingly, we showed that MC-mediated in situ–like DMS morphology is independent of myosin IIA and MKL1, pointing to a role of other mechanosensing elements such as microtubules or mechanosensitive channels, which could also be linked to actin dynamics.51,52
Importantly, our data showing that both myosin and MKL1 contribute to MC-mediated increased proplatelet formation but not to DMS morphology would suggest that DMS intracellular organization and the initiation of proplatelet extension are not directly related and could be uncoupled. This finding is in agreement with observations that in liquid culture, where the DMS does not adopt its in situ configuration, MKs are still able to extend proplatelets. Furthermore, the DMS appears to be highly dynamic, and our finding that it reorganizes upon cell resuspension raises the hypothesis that this type of membrane rearrangement is a normal step before proplatelet extension, when the stiffness of the medium decreases. Such conditions could be present when MKs reach the sinusoid vessels, and above all when a cytoplasmic protrusion comes into contact with the liquid environment of the blood. Whether this membrane rearrangement involves the physical separation of the DMS from the plasma membrane is unclear.53
It was shown a few years ago that the absence of myosin negatively affects the differentiation process and consequent proplatelet formation, provided that differentiation occurs in situ, where myosin is required to counteract unavoidable mechanical constraints.22 Here we show that myosin-deficient progenitors grown in an MC gel recover some features only observed in situ, especially a strong decrease in proplatelet formation and their abnormal morphology.21,22 In situ, myosin is required during MK maturation to counterbalance external local forces exerted by surrounding migrating cells. In MC gels, hydrophilic and hydrophobic interactions promote physical crosslinks with a finite lifetime. These crosslinks are transient in nature and heterogeneously distributed.54,55 Somehow similarly to the in situ BM, myosin is likely required to counterbalance and remodel the constraints generated by these crosslinks. In accordance with this idea, we observed that myosin IIA was distributed at the periphery but also within the cytoplasm of MKs grown in MC gels, like in situ, contrary to liquid culture (Figure 6A). It appears that in liquid culture with no stiffness, myosin IIA is dispensable for late maturation because no gross morphological differences are observed as compared with the WT, with no negative repercussions in terms of proplatelet formation. Furthermore, decreasing the RhoA/Rho kinase/myosin pathway in fully mature MKs favors proplatelet extension21,56-58 probably by releasing cortical tension.43,59
Among the mechanotransduction pathways recognized to date, MKL1 is well known to play a role in response to increasing substrate stiffness by regulating the transcription of a number of cytoskeletal genes.32,60 MKL1 nuclear relocation is also involved in MK differentiation.35 We showed that the MC gel promotes a greater amount of MKL1 nuclear translocation as compared with liquid conditions. Our finding that CCG-1423 inhibits proplatelet formation only when MKs are grown in an MC gel supports the hypothesis that MKL1 is relocated to the nucleus in response to the gel stiffness, where it will promote proplatelet formation. Although we have not investigated the sequence of events involved here, our data are in accordance with previous observations that MKL1 knockdown or inactivation decreases proplatelet and platelet formation,34,61 whereas MKL1 overexpression increases MK differentiation.33
In conclusion, we show in this work that re-creating 3D physical constraints like those progenitor cells may encounter in BM improves in vitro MK differentiation and proplatelet formation, in part through adaptations involving myosin IIA and MKL1. Thus, hydrogel-based 3D culture represents a more relevant system than liquid culture for the study of cell behavior during MK differentiation. Extended to human CD34+ cells, it could be an alternative way to evaluate samples from patients with abnormal platelet biogenesis related to unrecognized cytoskeletal alterations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Josiane Weber, Fabienne Proamer, and Patricia Laeuffer for excellent technical assistance; Emeline Aguilar for her help with data quantification; and Juliette Mulvihill for reviewing the English.
This study was supported by the Association de Recherche et Développement en Médecine et Santé Publique. F.P. was the recipient of a fellowship from the Société Française d’Hématologie, and A.A. was supported by a French government fellowship.
Authorship
Contribution: A.A. and F.P. designed and performed experiments, analyzed data, and wrote the paper; A.E., C.S., and D.C. performed and analyzed experiments; F.L. and C.G. discussed results and wrote the paper; and C.L. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Catherine Léon, UMR_S949 INSERM-Université de Strasbourg, Etablissement Français du Sang, 10, Rue Spielmann, B.P. N°36, 67065 Strasbourg Cedex, France; e-mail: catherine.leon@efs.sante.fr.
References
Author notes
A.A. and F.P. contributed equally to this study.