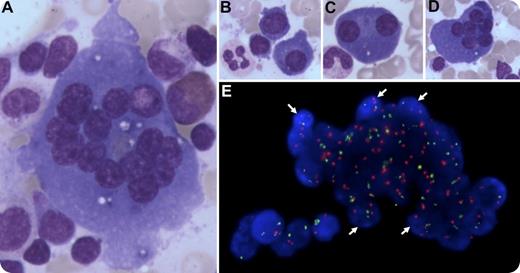
A 64-year-old man with immunoglobulin A κ multiple myeloma was treated initially with bortezomib, thalidomide, dexamethasone, and daratumumab, an anti-CD38 antibody. After 4 cycles, the monoclonal component had decreased from 27 g/L to 8 g/L. A bone marrow aspirate was performed. Our attention was caught by large multinucleated cells with basophilic cytoplasm (panel A; original magnification ×400, May Grünwald Giemsa stain) that could be identified at first as osteoclasts or dystrophic megakaryocytes. However, there was a continuum between dystrophic plasmocytes with 1, 2, or more nuclei and these giant multinucleated cells (panels B-D; original magnification ×200 for each panel, May Grünwald Giemsa stain). Using fluorescent in situ hybridization (red, chromosome 4; green, chromosome 14) and staining of the nuclei with 4′,6-diamidino-2-phenylindole (DAPI), we found that the nuclei of these cells harbored the cytogenetic abnormality observed at diagnosis (ie, a trisomy of chromosome 4; panel E, original magnification ×630, white arrows), confirming that they were indeed myelomatous polykaryons. Of note, these images were not seen on review of the diagnostic bone marrow.
Most of the time, multinucleated cells observed on a bone marrow smear are physiological, resulting from endomitosis (eg, megakaryocytes) or from fusion of mononucleated cells (eg, osteoclasts). However, one should keep in mind that multinucleated cells can also be part of a neoplastic process such as multiple myeloma. Although binucleated plasmocytes can be observed in healthy individuals, multinucleated plasmocytes must be considered as pathological.
A 64-year-old man with immunoglobulin A κ multiple myeloma was treated initially with bortezomib, thalidomide, dexamethasone, and daratumumab, an anti-CD38 antibody. After 4 cycles, the monoclonal component had decreased from 27 g/L to 8 g/L. A bone marrow aspirate was performed. Our attention was caught by large multinucleated cells with basophilic cytoplasm (panel A; original magnification ×400, May Grünwald Giemsa stain) that could be identified at first as osteoclasts or dystrophic megakaryocytes. However, there was a continuum between dystrophic plasmocytes with 1, 2, or more nuclei and these giant multinucleated cells (panels B-D; original magnification ×200 for each panel, May Grünwald Giemsa stain). Using fluorescent in situ hybridization (red, chromosome 4; green, chromosome 14) and staining of the nuclei with 4′,6-diamidino-2-phenylindole (DAPI), we found that the nuclei of these cells harbored the cytogenetic abnormality observed at diagnosis (ie, a trisomy of chromosome 4; panel E, original magnification ×630, white arrows), confirming that they were indeed myelomatous polykaryons. Of note, these images were not seen on review of the diagnostic bone marrow.
Most of the time, multinucleated cells observed on a bone marrow smear are physiological, resulting from endomitosis (eg, megakaryocytes) or from fusion of mononucleated cells (eg, osteoclasts). However, one should keep in mind that multinucleated cells can also be part of a neoplastic process such as multiple myeloma. Although binucleated plasmocytes can be observed in healthy individuals, multinucleated plasmocytes must be considered as pathological.
For additional images, visit the ASH IMAGE BANK, a reference and teaching tool that is continually updated with new atlas and case study images. For more information visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal