To the editor:
Immunoglobulin light chain amyloidosis (AL) with advanced cardiac involvement is characterized by short survival. Outcomes are especially poor for patients who have multiorgan involvement and coexisting multiple myeloma and do not achieve normalization of the serum free light chains with treatment (ie, fail to achieve hematologic complete remission).1,2
Treatment of AL includes the use of stem cell transplantation (SCT) in a minority (20% at Mayo Clinic) of patients with the majority being treated with proteasome inhibitor, alkylating agent, and/or steroid-based combinations.3,4 Patients with progressive/refractory disease after these treatments have a particularly poor outcome.
Daratumumab, an unconjugated, human anti-CD38 monoclonal antibody, produces high response rates including complete responses in dual refractory (immunomodulating agent and proteasome inhibitor) multiple myeloma patients. The rapid development and US Food and Drug Administration approval of daratumumab (2015) for patients with relapsed/refractory multiple myeloma offers a new first-in-class therapy.5 With its activity in myeloma, there is potential for activity in AL; however, the safety of daratumumab in advanced AL is unknown. We report the first use of daratumumab in 2 patients with multiple myeloma whose disease was complicated by advanced multisystem AL.
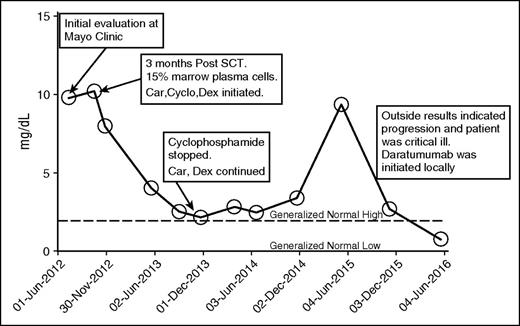
In case 1 (Table 1), a 62-year-old white male was referred to the Mayo Clinic for consideration of autologous hematopoietic SCT for progressive hepatic and cardiac κ light chain AL. A year prior to referral, he was diagnosed with International Staging System stage III immunoglobulin G κ multiple myeloma (50% plasmacytosis; calcium, 15.3 mg/dL; β-2 microglobulin, 9.1 mg/L; creatinine, 2.1 mg/dL) with vertebral fractures. After discontinuing a trial of lenalidomide because of severe skin toxicity, he achieved a brief partial remission following 8 months of cyclophosphamide, bortezomib, and dexamethasone therapy. Prior to presenting to the clinic, he developed rapidly progressive hepatomegaly. Alkaline phosphatase was 422 U/L, and a liver biopsy demonstrated extensive amyloid deposition. Cardiac involvement was confirmed with magnetic resonance imaging with an interventricular septal thickness of 16 mm. Brain natriuretic peptide was 1483 (pg/mL), and cardiac troponin T was 0.08 (ng/mL). The patient underwent reduced-dose melphalan conditioning (100 mg/m2) and autologous stem cell rescue. SCT was uneventful; however, he did not achieve a very good partial response, and at day 100, treatment with carfilzomib, cyclophosphamide, and dexamethasone was started. After 9 months of therapy, cyclophosphamide was stopped as the light chain level plateaued after achieving very good partial response and the patient developed progressive anemia. In December 2014, he developed biochemical progression, and cyclophosphamide was added with an initial response for 6 months that was followed by rapid progression and clinical deterioration (December 2015). He developed rectal bleeding because of friable hemorrhoids and mucosal vascular involvement with amyloid. He required multiple hospitalizations for congestive heart failure and multiple surgical interventions to control rectal bleeding resulting in transfusion-dependent anemia. Daratumumab treatment was initiated as per the US prescribing information with the first dose given as an inpatient, which he tolerated well. At the conclusion of 8 weekly treatments, there was improvement in his performance status and the bleeding subsided resulting in transfusion independence. It was the first time in 4 years of illness that he achieved normalization of serum free light chains (Figure 1). NT-proBNP went from 2882 (pg/mL) to 3568 (pg/mL), and cardiac troponin T declined from 0.05 ng/mL to <0.01 ng/mL. Alkaline phosphatase increased mildly, but liver size remained stable.
Disease and treatment characteristics for cases 1 and 2
| Disease/treatment characteristics* . | Case 1 . | Case 2 . | ||
|---|---|---|---|---|
| Predaratumumab . | Postdaratumumab . | Predaratumumab . | Postdaratumumab . | |
| Creatinine (mg/dL) | 1.9 | 1.4 | 3.1 | 2.0 |
| NT-ProBNP (pg/mL) | 2882 | 3568 | 1451 | 1660 |
| Troponin T (ng/mL) | 0.05 | <0.01 | 0.05 | 0.03 |
| Alkaline phosphatase (U/L) | 430 | 582 | 61 | 73 |
| Electrocardiogram | Pseudo-infarct pattern | NA | Normal | NA |
| Echocardiogram | ||||
| EF (%) | 64 | NA | 68 | NA |
| IVS (cm) | 1.6 | 1.1 | ||
| LV regional strain (normal is more negative than −18) | −7 | −23 | ||
| Prior treatment | ||||
| SCT | Yes | Yes | ||
| Bortezomib | Yes | Yes | ||
| IMiD | Yes | Yes | ||
| Dexamethasone | Yes | Yes | ||
| Carfilzomib | Yes | Yes | ||
| Disease/treatment characteristics* . | Case 1 . | Case 2 . | ||
|---|---|---|---|---|
| Predaratumumab . | Postdaratumumab . | Predaratumumab . | Postdaratumumab . | |
| Creatinine (mg/dL) | 1.9 | 1.4 | 3.1 | 2.0 |
| NT-ProBNP (pg/mL) | 2882 | 3568 | 1451 | 1660 |
| Troponin T (ng/mL) | 0.05 | <0.01 | 0.05 | 0.03 |
| Alkaline phosphatase (U/L) | 430 | 582 | 61 | 73 |
| Electrocardiogram | Pseudo-infarct pattern | NA | Normal | NA |
| Echocardiogram | ||||
| EF (%) | 64 | NA | 68 | NA |
| IVS (cm) | 1.6 | 1.1 | ||
| LV regional strain (normal is more negative than −18) | −7 | −23 | ||
| Prior treatment | ||||
| SCT | Yes | Yes | ||
| Bortezomib | Yes | Yes | ||
| IMiD | Yes | Yes | ||
| Dexamethasone | Yes | Yes | ||
| Carfilzomib | Yes | Yes | ||
EF, ejection fraction; IMiD, immunomodulatory drugs; IVS, interventricular septum thickness; LV, left ventricular; NA, not available; NT-ProBNP, N-terminal prohormone of brain natriuretic peptide.
The laboratory data in both cases from Mayo Clinic are presented, and the pre- and postdaratumumab values are separated by several months, as both patients were treated at different facilities.
Kappa free light chain. Car, carfilzomib; Cyclo, cyclophosphamide; Dex, dexamethasone.
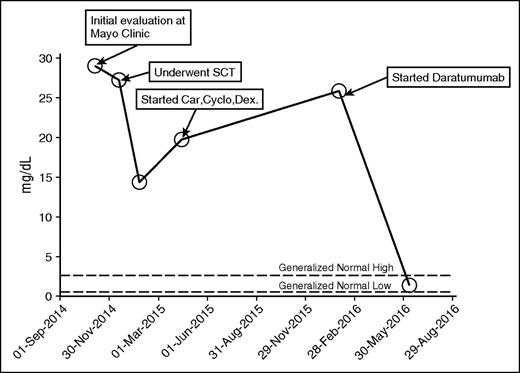
Kappa free light chain. Car, carfilzomib; Cyclo, cyclophosphamide; Dex, dexamethasone.
In case 2 (Table 1), a 55-year-old white male was diagnosed with λ light chain amyloidosis as he presented with unexplained nephrotic syndrome with more than 10 g/d albuminuria. He was treated with 8 months of cyclophosphamide, bortezomib, and dexamethasone. At baseline, he had 15% marrow involvement by λ restricted plasma cells. He responded well to treatment (data not available), but it was discontinued because of severe peripheral neuropathy. His proteinuria progressed over the course of 6 to 8 months, and he presented to the Mayo Clinic. After extensive evaluation, high-dose therapy and SCT were recommended for λ light chain AL with kidney involvement and progressive proteinuria. The patient tolerated high-dose melphalan (200 mg/m2) well except for fluid overload associated with stem cell mobilization. He achieved partial response (50% reduction in λ light chain) after SCT. Six months after transplant, he developed increasing proteinuria and λ light chains increased; salvage treatment with carfilzomib, cyclophosphamide, and dexamethasone was initiated. After 6 months of therapy, he developed progressive disease and worsening peripheral neuropathy. At this time, treatment with daratumumab was started. After 12 weeks of treatment, the patient reported significant improvement in quality of life with improvement in renal function (serum creatinine decreased from 3.1 mg/dL to 2.0 mg/dL) and edema (serum albumin increased from 1.6 mg/dL to 2.3 mg/dL). He achieved hematologic complete remission (Figure 2). He developed a mild infusion reaction (burning sensation in eye and scratchy feeling in throat) with the first infusion that was given over 15 hours, but each subsequent infusion was shorter, most recently 3 to 4 hours.
Our patients demonstrate that daratumumab lowers the light chain and is able to do so rapidly. Second, it can be used in the dose and schedule that is approved for multiple myeloma. The latter is very important in patients with advanced cardiac AL because of concerns about volume overload and infusion reaction-related morbidity. Our first patient had advanced cardiac and hepatic AL but was able to tolerate the weekly regimen with daratumumab. Although it is early to gauge organ response, the fact that the patients’ serum free light chain levels normalized for the first time in the course of these 2 patients’ illnesses despite prior use of proteasome inhibitors, immunomodulatory drugs, and SCT is very encouraging. We believe that this patient experience justifies a formal clinical trial evaluating the safety and efficacy of daratumumab in AL, a disease with a mortality of 40% at 1 year.
Authorship
Contribution: T.S. designed the study and wrote the manuscript; B.F. wrote and reviewed the manuscript; A.A. performed patient care and reviewed the manuscript; and M.A.G. wrote and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Taimur Sher, Mayo Clinic, 4500 San Pablo Rd, Jacksonville, FL 32224; e-mail: sher.taimur@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal