Key Points
FcμR is a more selective target for the CAR T-cell therapy of CLL compared with currently used targets, including CD19.
Abstract
Adoptive cell therapy of chronic lymphocytic leukemia (CLL) with chimeric antigen receptor (CAR)–modified T cells targeting CD19 induced lasting remission of this refractory disease in a number of patients. However, the treatment is associated with prolonged “on-target off-tumor” toxicities due to the targeted elimination of healthy B cells demanding more selectivity in targeting CLL cells. We identified the immunoglobulin M Fc receptor (FcμR), also known as the Fas apoptotic inhibitory molecule-3 or TOSO, as a target for a more selective treatment of CLL by CAR T cells. FcμR is highly and consistently expressed by CLL cells; only minor levels are detected on healthy B cells or other hematopoietic cells. T cells with a CAR specific for FcμR efficiently responded toward CLL cells, released a panel of proinflammatory cytokines and lytic factors, like soluble FasL and granzyme B, and eliminated the leukemic cells. In contrast to CD19 CAR T cells, anti-FcμR CAR T cells did not attack healthy B cells. T cells with anti-FcμR CAR delayed outgrowth of Mec-1-induced leukemia in a xenograft mouse model. T cells from CLL patients in various stages of the disease, modified by the anti-FcμR CAR, purged their autologous CLL cells in vitro without reducing the number of healthy B cells, which is the case with anti-CD19 CAR T cells. Compared with the currently used therapies, the data strongly imply a superior therapeutic index of anti-FcμR CAR T cells for the treatment of CLL.
Introduction
Despite extensive clinical exploration, chronic lymphocytic leukemia (CLL) is still hard to treat; allogeneic hematopoietic stem cell transplantation is currently the only curative treatment option.1,2 The first-line treatment of elderly patients is a combined chemoimmunotherapy with a therapeutic anti-CD20 antibody and an alkylating agent, such as chlorambucil or bendamustine3-5 ; for younger patients, the first-line treatment is the combination of fludarabine, cyclophosphamide, and rituximab.6,7 Despite high initial response rates and second-line treatment options with the Bruton’s tyrosine kinase inhibitor ibrutinib8 and the PI3K inhibitor idelalisib,9 a substantial number of patients relapse with the outgrowth of resistant cells and refractory disease.10
In this situation, adoptive cell therapy with patients’ own cytolytic T cells specifically redirected toward their CLL cells provides a therapeutic option for inducing complete remission in the long term. The T cells are ex vivo engineered with a CD19-specific chimeric antigen receptor (CAR), which is composed of an extracellular single-chain fragment of variable region (scFv) antibody for CD19 binding and an intracellular signaling domain for T-cell activation, frequently the TCR CD3ζ domain linked to a costimulatory domain like CD28 or 4-1BB. Such engineered T cells are capable of migrating through tissues, amplifying upon stimulation, killing target cells in a repetitive fashion, establishing a specific and lasting memory, and secreting proinflammatory cytokines, which in turn attract other immune cells to combat the leukemia. Early phase trials with anti-CD19 CAR T cells showed particular success in the treatment of refractory CLL and the induction of a lasting remission in 27% of patients being treated.11 Current cell therapy with anti-CD19 CAR T cells, however, produces substantial “on-target off-tumor” toxicities due to the targeted elimination of healthy B cells. Accordingly, patients treated with anti-CD19 CAR T cells suffer from B-cell aplasia and hypo-gammaglobulinemia for years, requiring repetitive immunoglobulin substitutions. A more beneficial discrimination between healthy and malignant B cells is required to protect the patient from treatment-associated B-cell aplasia in the long term.
We identified the immunoglobulin M (IgM) Fc receptor (FcμR) as an alternative target for CAR T-cell therapy of CLL with more selective killing of leukemia cells while leaving healthy B cells largely untouched. FcμR, also known as Fas apoptotic inhibitory molecule-3 (FAIM3) or TOSO, is a transmembrane protein with a restricted expression profile to hematopoietic and lymphoid tissues and a pronounced overexpression by CLL cells.12-14 We here report an FcμR-specific CAR that redirects and activates patient’s T cells with high specificity and selectivity toward CLL cells while sparing healthy B cells. Data support T-cell therapy with the anti-FcμR CAR for a more selective treatment with a superior therapeutic index compared with the currently used anti-CD19 CAR T cells.
Materials and methods
Blood samples, cell lines, and reagents
All studies involving human blood cells were approved by the Institutional Review Board of the University Hospital of Cologne (reference no. 01-090 and 11-319). The study was performed according to the Helsinki protocol and approved by the Ethics Committee of the University of Cologne. Human T cells were isolated from the peripheral blood of healthy donors by density centrifugation and stimulated by the agonistic anti-CD3 antibody OKT3 (200 ng/mL), interleukin-2 (IL-2; 500 U/mL), and the agonistic anti-CD28 antibody 15E8 (50 ng/mL) for 48 hours. Primary human CLL B cells were isolated and purified as described before.15 To obtain healthy B cells, peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by density gradient centrifugation, and the CD19+ B-cell fraction was purified using CD19 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Trypan blue staining was used to assess viability. 293T cells (American Type Culture Collection CRL-11268) are human embryonic kidney cells that express the SV40 large T antigen. 293 cells are human embryonic kidney cells (American Type Culture Collection CRL-1573). FcμR+ 293 cells were derived from 293 cells by stable transfection with plasmid DNA encoding the full-length FcμR protein. Mec-1 cells are human chronic B-cell leukemia-derived cells (Deutsche Sammlung von Mikroorganismen und Zellkulturen ACC 497). The anti-FcμR monoclonal antibody (mAb) hybridoma cell line that produces the mAb 6B10 was described earlier.12 Primary human T cells and Mec-1 cells were cultured in RPMI 1640 medium (Invitrogen Life Technologies, Karlsruhe, Germany) containing 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM l-glutamine, and 10% (vol/vol) fetal calf serum (FCS; Invitrogen Life Technologies). 293T, 293, and FcμR+ 293 cells were cultured in Dulbecco’s modified Eagle medium (Invitrogen Life Technologies) containing 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM l-glutamine, and 10% (vol/vol) FCS. The anti-FcμR hybridoma cell line was cultured in RPMI 1640 medium, containing 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM l-glutamine, and 20% (vol/vol) heat-inactivated FCS. OKT3 and 15E8 mAbs were affinity purified from hybridoma supernatants using goat anti-mouse IgG1 antibody (Southern Biotechnology, Birmingham, AL) immobilized on N-hydroxysuccinimide ester–activated sepharose (Amersham Biosciences, Freiburg, Germany). The fluorescein isothiocyanate (FITC)-conjugated anti-CD3 mAb, the phycoerythrin (PE), and the FITC-conjugated anti-CD19 mAb, and the PE-conjugated mouse IgG1 mAb were purchased from Miltenyi Biotec. The goat anti-human IgG antibody and its biotin- and PE-conjugated F(ab′)2 fragment derivatives were purchased from Southern Biotechnology. The allophycocyanin (APC)-conjugated anti-CD5 mAb, APC-conjugated Annexin V, the PE-conjugated anti-FcµR mAb (clone HM14), and the FITC-conjugated rat IgG2a mAb were purchased from BD Bioscience (San Jose, CA). The FITC-conjugated anti-FcµR mAb (clone 6B10) was purchased from Genovac (Freiburg, Germany). The anti-FAIM3 mAb (clone 1E4) was purchased from Abnova (Taipei City, Taiwan). The anti-FAIM3 mAb was purchased from Abcam (Cambridge, UK). The PE-conjugated mouse IgG2b mAb was purchased from Biolegend (London, UK). The anti-human interferon-γ (IFN-γ) antibody NIB42, the biotinylated anti-human IFN-γ antibody 4S.B3, the anti-human IL-2 antibody 5344-111, and the biotinylated anti-human IL-2 antibody B33-2 were purchased from BD Bioscience. Recombinant human IL-2 was purchased from Novartis (Basel, Switzerland), carboxyfluorescein succinimidyl ester (CFSE) from Invitrogen, and calcein-AM from Thermo Fisher Scientific (Waltham, MA).
Engineering of the anti-FcμR CAR and anti-FcμR-Fc protein
The generation of the anti-CD19 CAR retroviral vector was described earlier.16 To engineer the anti-FcμR scFv, RNA from 6B10 hybridoma cells was isolated using Trizol, reverse transcribed using the “Transcriptor First Strand cDNA Synthesis Kit ” (Roche Diagnostics, Mannheim, Germany) and the oligonucleotide primers (VH: #765; VL: #884, #885; Table 1). VH and VL chain complementary DNA (cDNA) was amplified by polymerase chain reaction (PCR) (GenBank accession numbers: KX353917 and KX353918). The first-strand cDNA was poly-G-tailed using the terminal transferase and deoxyguanosine triphosphates following a PCR with antisense nested primers and a sense primer with a poly-C sequence (VH: #766, #768; VL: #877, #878, #768) according to published procedures.17 Purified PCR products were submitted to a second PCR using primers that were flanked by the restriction site NcoI and overlapping complementary sequences encoding the Igκ leader 5′ to the VH (#777, #778) and the restriction site BamHI and the overlapping complementary sequences encoding the (Gly4Ser)3 linker to the 3′VL and the 5′VL (#892, #893), respectively. Equimolar amounts of VH and VL cDNA were annealed by their complementary ends and PCR amplified by using the 5′VH primer flanked by the NcoI site and the 3′VL primer flanked by the BamHI site (#782, #893). The produced DNA coding for VH-(Gly4Ser)3-VL was purified and digested by NcoI and BamHI and inserted into the plasmid DNA pBullet-Lκ-BW431/26scFv-Fc-CD28-CD3ζ (anticarcinoembryonic antigen [CEA] CAR) replacing the CEA-specific scFv. Both DNA fragments were ligated to generate the new plasmid pBullet-Lκ-anti-FcμR-scFv-Fc-CD28-CD3ζ (anti-FcμR CAR). To engineer the anti-FcμR scFv-Fc CAR extracellular domain, the pBullet-Lκ-anti-FcμR-scFv-Fc-CD28-CD3ζ DNA (anti-FcμR CAR) was digested by BamHI and XhoI, and the DNA coding for anti-FcμR-scFv was isolated. The DNA coding for IgG1 Fc was isolated from pRSV-Lκ-anti-CD30-Fc DNA by BamHI and XhoI digestion. The 2 DNA fragments were ligated to generate the anti-FcμR-Fc plasmid pBullet-Lκ-anti-FcμR-scFv-Fc. The anti-CEA scFv-Fc encoding plasmid was described earlier.18 To produce the scFv-Fc antibodies, which represent the extracellular domain of the CAR, 293T cells were transfected with the expression plasmid coding for the anti-FcμR scFv-Fc or the anti-CEA scFv-Fc antibody, respectively. After 48 hours, culture supernatants were harvested and cleared by centrifugation at 1500g for 15 minutes. The amount of scFv-Fc in the supernatants was determined by enzyme-linked immunosorbent assay (ELISA), and equimolar amounts of scFv-Fc were incubated for 1 hour on ice with FcμR+ or FcµR− 293 cells, Mec-1 cells, CLL cells, or healthy CD3+ T or CD19+ B cells (2 × 105 cells/tube). Bound antibodies were detected by flow cytometry using a PE-conjugated F(ab′)2 goat anti-human IgG antibody (1 μg/mL). Unspecific binding of the PE-labeled anti-human antibody was blocked by preincubating the cells for 30 minutes on ice with an unlabeled goat anti-human antibody (10 µg/mL).
Oligonucleotide primers used for engineering the anti-FcμR-scFv-Fc-CD28-CD3ζ and anti-FcμR-scFv-Fc
| Number . | Orientation . | Name . | 3′-5′ Sequence . |
|---|---|---|---|
| 765 | Antisense | rIgG2ab-1 | AATTTTCTTGTCCACCTTGG |
| 884 | Antisense | CL1FOR | ACACTCAGCACGGGACAAACTCTTCTC |
| 885 | Antisense | CL2FOR | ACACTCTGCAGGAGACAGACTCTTTTC |
| 766 | Antisense | HBS-rG2a | CGTCATGTCGACGGATCCAAGCTTAATAGCCCTTGACCAGGCAT |
| 768 | Sense | rIgG-anc | CGTCGATGAGCTCTAGAATTCGCATGTGCAAGTCCGATG |
| GTCCCCCCCCCCCCCC | |||
| 877 | Antisense | rIgCl-1 | TGTCACCATACACACCAGTGTAGCC |
| 878 | Antisense | rIgCl-2 | ATCAGAAATCAGACACACCAGTGTGGCT |
| 777 | Antisense | rev_pcR2.1_rVh | TGAGGAGACTGTGACCATGACTCCT |
| 778 | Sense | for_pcR2.1_rVh | AGACTGCCATGGATTTTCAGGTGCAGATTTTCAGCTTCCTGCTAAT |
| CAGTGCCTCAGTCATAATGTCTAGAGAGGTGCAGCTCGTGGAAACAG | |||
| 892 | Sense | FcμRla-for | CGGAGTCATGGTCACAGTCTCCTCAGGAGGTGGTGGATCGGGCGGT |
| GGCGGGTCGGGTGGCGGCGGATCTAGCTATGAGCCTGATCCAACCACCTTC | |||
| 893 | Antisense | FcμRla-rev | CTTATCGGATCCCCTAGGACAGTGAGCTTGGTTCC |
| 782 | Sense | for-flank-assVh | AGACTGCCATGGATTTTCAGGTGCAGATTTTCAGCTTCCTGCTAA |
| Number . | Orientation . | Name . | 3′-5′ Sequence . |
|---|---|---|---|
| 765 | Antisense | rIgG2ab-1 | AATTTTCTTGTCCACCTTGG |
| 884 | Antisense | CL1FOR | ACACTCAGCACGGGACAAACTCTTCTC |
| 885 | Antisense | CL2FOR | ACACTCTGCAGGAGACAGACTCTTTTC |
| 766 | Antisense | HBS-rG2a | CGTCATGTCGACGGATCCAAGCTTAATAGCCCTTGACCAGGCAT |
| 768 | Sense | rIgG-anc | CGTCGATGAGCTCTAGAATTCGCATGTGCAAGTCCGATG |
| GTCCCCCCCCCCCCCC | |||
| 877 | Antisense | rIgCl-1 | TGTCACCATACACACCAGTGTAGCC |
| 878 | Antisense | rIgCl-2 | ATCAGAAATCAGACACACCAGTGTGGCT |
| 777 | Antisense | rev_pcR2.1_rVh | TGAGGAGACTGTGACCATGACTCCT |
| 778 | Sense | for_pcR2.1_rVh | AGACTGCCATGGATTTTCAGGTGCAGATTTTCAGCTTCCTGCTAAT |
| CAGTGCCTCAGTCATAATGTCTAGAGAGGTGCAGCTCGTGGAAACAG | |||
| 892 | Sense | FcμRla-for | CGGAGTCATGGTCACAGTCTCCTCAGGAGGTGGTGGATCGGGCGGT |
| GGCGGGTCGGGTGGCGGCGGATCTAGCTATGAGCCTGATCCAACCACCTTC | |||
| 893 | Antisense | FcμRla-rev | CTTATCGGATCCCCTAGGACAGTGAGCTTGGTTCC |
| 782 | Sense | for-flank-assVh | AGACTGCCATGGATTTTCAGGTGCAGATTTTCAGCTTCCTGCTAA |
T-cell modification and flow cytometry
Human peripheral blood T cells were retrovirally transduced to express the CARs as described earlier.19 The CAR was monitored by flow cytometry using a PE-conjugated F(ab′)2 goat anti-human IgG antibody and a FITC-conjugated mouse anti-human CD3 antibody. FcμR was recorded using the FITC-conjugated anti-FcμR mAb 6B10, the PE-conjugated anti-FcµR mAb HM14, anti-FAIM3 mAb followed by the PE-conjugated anti-mouse IgG2b antibody, anti-FAIM3 mAb 1E4 followed by the PE-conjugated anti-mouse IgG2b antibody, or the appropriate isotype-matched control antibodies. Freshly isolated CLL cells or healthy donor PBMCs were additionally stained with an APC- or FITC-conjugated mouse anti-human CD5 antibody and a PE-conjugated mouse anti-human CD19 antibody. Data were recorded using a FACSCanto II cytofluorometer equipped with FACS-Diva software (Becton Dickinson, Mountain View, CA) and FlowJo software 7.5 (Treestar, Ashland, OR).
Cytotoxicity assays
Human T cells were engineered with the respective CAR, cultivated in medium without stimulation for 24 hours, and coincubated in increasing numbers with target cells (1.5 × 104 cells/well) for 48 hours in 96-well round-bottomed plates. Specific lysis of CAR T cells was monitored by a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt (XTT)-based colorimetric assay (“Cell Proliferation Kit II”; Roche Diagnostics). Reduction of XTT to formazan by viable tumor cells was colorimetrically monitored. Maximal reduction of XTT was determined as the mean of 12 wells containing tumor cells only and the background as the mean of 12 wells containing medium. Nonspecific formation of formazan due to the presence of T cells was determined from triplicate wells containing T cells in the same number as in the corresponding experimental wells. Specific lysis was calculated as follows: specific lysis (%) = (1 − optical density [experimental wells − corresponding number of T cells]/optical density (tumor cells without T cells − medium)] × 100. In an alternative assay, target cells (106/mL) were incubated with 10 µM calcein-AM for 30 minutes at 37°C, washed twice, and coincubated (1.5 × 104 cells/well) in 96-well round-bottomed plates for 4 hours with T cells at various E:T cell ratios. Supernatant (100 µL) was transferred into a black, white-bottomed 96-well plate, and the fluorescence was recorded at 492-nm excitation and 520-nm emission wavelength. Target cells in the presence of 2% (vol/vol) Triton X-100 were used to record maximum calcein release. Specific lysis was calculated as follows: specific lysis (%) = (experimental release – spontaneous release)/(maximum release – spontaneous release) × 100.
Cytokine detection
Cell culture supernatants were analyzed for IFN-γ and IL-2 release by ELISA using the matched pair antibodies NIB42 and B133.5 for IFN-γ or the matched pair antibodies 5344-111 and B33-2 for IL-2. Briefly, IFN-γ was bound to the solid-phase mAb NIB42 and detected by the biotinylated mAb B133. IL-2 was bound to the solid-phase bound mAb B33-2 and detected by the biotinylated anti-human IL-2 mAb. The reaction product was visualized by a peroxidase-streptavidin conjugate and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (Roche Diagnostics) as substrate.
Competition assay
FcµR+ 293 cells (0.5 × 106 cells) were incubated in a 20-fold excess with the unlabeled competitor mAb (20 µg/mL) for 30 minutes on ice. Thereafter, cells were incubated with the fluorochrome-conjugated mAb (1 µg/mL) for 30 minutes and the fluorescence was recorded by flow cytometry. An isotype-matched unlabeled antibody served as control. Mean fluorescence intensity (MFI) of the fluorochrome-conjugated mAb without competitor was set as 100% (ie, maximal binding) and the competition was calculated as follows: competition (%) = 100 – maximal binding.
Soluble FcµR
Soluble FcµR-Fc was produced by 293T cells transfected with plasmid DNA encoding FcµR-Fc. Anti-FcµR CAR T cells were preincubated with serial dilutions of FcµR-Fc protein for 30 minutes followed by incubation with FcµR+ 293 target cells (1.5 × 104 cells) in an effector-to-tumor cell ratio of 1.5:1 for 48 hours. Specific lysis was monitored by an XTT-based viability assay.
CFSE labeling
Freshly isolated CLL or healthy B cells were labeled with CFSE. Briefly, cells were washed twice in phosphate-buffered saline (PBS), incubated with CFSE labeling solution (0.83 μM final concentration) for 5 minutes on ice, washed 4 times with cold RPMI 1640 medium, 10% (vol/vol) FCS, and incubated for 30 minutes on ice. Cells were washed twice in RPMI 1640 medium, 10% (vol/vol) FCS.
Annexin V staining
CFSE-labeled CLL or healthy B cells (105 cells) were coincubated with CAR T cells (5 × 105 cells) for 24 hours, washed twice in cold PBS, and labeled with Annexin V-APC in binding buffer according to the manufacturer’s protocol (“Annexin V-APC apoptosis detection kit”; BD Bioscience). Data were recorded by flow cytometry, and the number of CFSE-labeled, Annexin V–positive target cells was determined.
Multiplex analysis of culture supernatants
The human cytokines, IL-2, IL-4, IL-10, IL-6, IL-17A, tumor necrosis factor-α (TNF-α), IFN-γ, and the cytolysis-associated proteins, sFas, sFasL, granzyme A, granzyme B, perforin, and granulysin, were detected in the culture supernatants after coincubation of CAR T cells with the autologous CLL cells using the “LEGENDplex Human CD8 Panel” bead-based immunoassay (BioLegend, San Diego, CA) according to the manufacturer’s protocol. Data were recorded by a FACSCanto II cytofluorometer (Becton Dickinson) and analyzed using the “LEGENDplex” Data Analysis software 7.0.
Mouse studies
Groups of 16- to 20-week-old Rag2−/− cγ−/− mice (#4111; Taconic, Cologne, Germany) (n = 7 per group) were intravenously injected with 5 × 106 Mec-1 cells and 3 × 106 CAR T cells or without T cells for control. Mice were inspected every 1 to 3 days for neurological symptoms resulting from expansion of injected Mec-1 cells. Upon first appearance of disease signs, mice were sacrificed according to the Protection of Animals Act. Results were presented as disease-free survival, and significant differences were determined utilizing the Log-rank test.
Results
FcμR is a candidate surface antigen for selectively targeting CLL cells
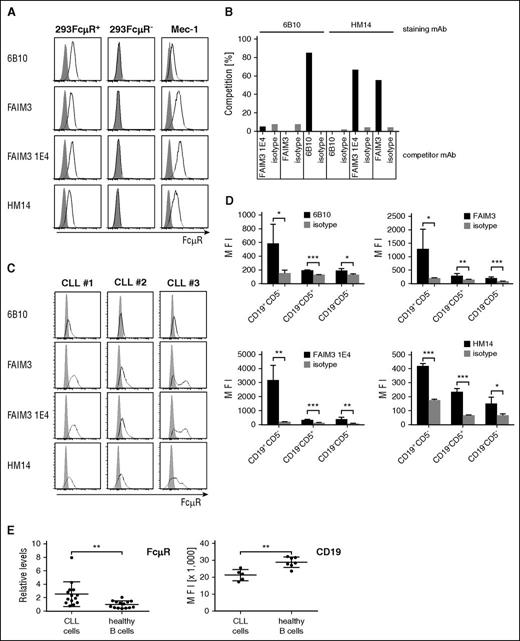
Current CAR T-cell therapy of CLL targets CD19 or CD20, which both are likewise expressed by healthy B cells; as a consequence, patients suffer from a lasting B-cell aplasia after adoptive therapy with cytolytic CAR T cells. We therefore screened for a more CLL-selective antigen and revealed FcμR, also known as TOSO or FAIM3, as a potential target. FcμR was detected by the mAb 6B10 on cells of the leukemic Mec-1 cell line; binding by the 6B10 antibody is specific because it detects FcμR on transfected 293 cells but not on nonmodified 293 cells (Figure 1A). The FcμR-specific antibodies, FAIM3, FAIM3 1E4, and HM14, also react with the respective FcμR+ 293 cells. Binding of the 6B10 antibody to FcμR+ cells is not blocked by the tested anti-FcμR antibodies (Figure 1B), indicating that the 6B10 mAb recognizes a different FcμR epitope than the other antibodies. For comparison, the FAIM3 and FAIM3 1E4 antibodies compete in binding with the HM14 antibody, indicating identical or overlapping binding epitopes. Patients’ CD19+CD5+ CLL cells react with the 6B10 antibody as with the other tested anti-FcμR antibodies (Figure 1C). In the blood of healthy donors, there is some marginal FcµR expression by healthy CD19+ CD5− B cells and far less by CD19− CD5+ T cells (Figure 1D). However, FcµR is expressed by CLL cells at higher levels than by healthy B cells. In contrast to FcµR, the expression level of CD19 is higher on healthy B cells compared with CLL cells (Figure 1E). Based on the favorite expression level on CLL cells vs healthy B cells, we conclude that FcμR may be a suitable surface antigen for more selective CLL targeting while sparing the patient’s B cells.
FcμR is highly expressed by CLL cells compared with healthy B and T cells. FcµR (FAIM3) is detected on the surface of FcµR-positive cell lines, healthy donor PBMCs, and patients’ CLL cells by mAbs. (A) Cell line 293 was genetically modified to express FcμR; cells (FcµR+ 293, FcµR—293, and Mec-1; 0.5 × 106 cells each) were stained with the anti-FcµR mAb (clone 6B10), anti-FAIM3 mAb, anti-FAIM3 mAb (clone 1E4), and the anti-FcµR mAb (clone HM14) (bold lines) or the respective isotype-matched control antibodies (gray histograms). (B) The anti-FcµR mAb 6B10 does not compete in binding with the other anti-FcμR mAbs. FcµR+ 293 cells were incubated with a 20-fold excess of the competitor antibody, stained with the labeled staining antibody as indicated, and recorded by flow cytometry. Competition was calculated as follows: competition (%) = 100 – maximal binding. The 6B10 mAb does not compete in binding with the FAIM3, FAIM3 1E4, or HM14 antibody, whereas HM14 competes with the FAIM3 and the FAIM3 1E4 mAb. (C) CLL cells from 3 patients (#1, #2, #3) were recorded for FcμR by flow cytometry. Isolated cells (0.5 × 106 cells) were stained with the anti-CD5 mAb and the anti-CD19 mAb. CD19+CD5+ CLL cells were analyzed for FcµR by various anti-FcµR antibodies (bold line) or isotype-matched control antibodies (gray histograms). (D) FcµR is preferentially expressed by healthy CD19+ CD5− B cells and less by CD19− CD5+ T cells. PBMCs from healthy donors (n = 4; 0.5 × 106 cells) were stained with the anti-CD5 mAb and the anti-CD19 mAb. The cell subpopulations (CD19+CD5−, CD19−CD5+, CD19−CD5−) were recorded for FcµR using the respective antibodies. Isotype-matched control antibodies served as control. Bars represent the MFI of FcµR staining. Statistic analysis was performed using the Student t test (*P < .05; **P < .01; ***P < .001). (E) CLL cells (n = 14) and healthy donor B cells (n = 14) were recorded by flow cytometry for FcμR by staining with the mAb 6B10. The FcμR MFI on healthy B cells was set to 1, and the respective FcμR levels were calculated (relative levels). CD19 was recorded on CLL cells (n = 5) and healthy donor B cells (n = 7), and the MFI was shown. Statistical analysis was performed using the Student t test (FcμR, **P = .0051; CD19, **P = .002).
FcμR is highly expressed by CLL cells compared with healthy B and T cells. FcµR (FAIM3) is detected on the surface of FcµR-positive cell lines, healthy donor PBMCs, and patients’ CLL cells by mAbs. (A) Cell line 293 was genetically modified to express FcμR; cells (FcµR+ 293, FcµR—293, and Mec-1; 0.5 × 106 cells each) were stained with the anti-FcµR mAb (clone 6B10), anti-FAIM3 mAb, anti-FAIM3 mAb (clone 1E4), and the anti-FcµR mAb (clone HM14) (bold lines) or the respective isotype-matched control antibodies (gray histograms). (B) The anti-FcµR mAb 6B10 does not compete in binding with the other anti-FcμR mAbs. FcµR+ 293 cells were incubated with a 20-fold excess of the competitor antibody, stained with the labeled staining antibody as indicated, and recorded by flow cytometry. Competition was calculated as follows: competition (%) = 100 – maximal binding. The 6B10 mAb does not compete in binding with the FAIM3, FAIM3 1E4, or HM14 antibody, whereas HM14 competes with the FAIM3 and the FAIM3 1E4 mAb. (C) CLL cells from 3 patients (#1, #2, #3) were recorded for FcμR by flow cytometry. Isolated cells (0.5 × 106 cells) were stained with the anti-CD5 mAb and the anti-CD19 mAb. CD19+CD5+ CLL cells were analyzed for FcµR by various anti-FcµR antibodies (bold line) or isotype-matched control antibodies (gray histograms). (D) FcµR is preferentially expressed by healthy CD19+ CD5− B cells and less by CD19− CD5+ T cells. PBMCs from healthy donors (n = 4; 0.5 × 106 cells) were stained with the anti-CD5 mAb and the anti-CD19 mAb. The cell subpopulations (CD19+CD5−, CD19−CD5+, CD19−CD5−) were recorded for FcµR using the respective antibodies. Isotype-matched control antibodies served as control. Bars represent the MFI of FcµR staining. Statistic analysis was performed using the Student t test (*P < .05; **P < .01; ***P < .001). (E) CLL cells (n = 14) and healthy donor B cells (n = 14) were recorded by flow cytometry for FcμR by staining with the mAb 6B10. The FcμR MFI on healthy B cells was set to 1, and the respective FcμR levels were calculated (relative levels). CD19 was recorded on CLL cells (n = 5) and healthy donor B cells (n = 7), and the MFI was shown. Statistical analysis was performed using the Student t test (FcμR, **P = .0051; CD19, **P = .002).
An anti-FcμR CAR for redirecting T cells
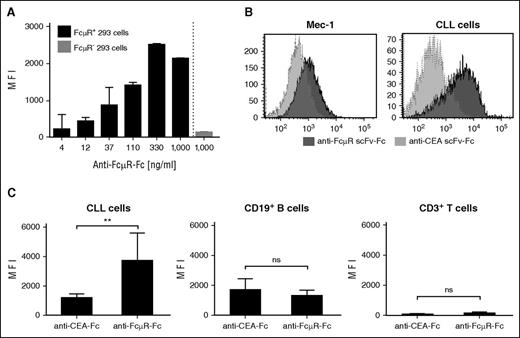
To specifically target FcμR, we converted the rat mAb 6B10 into an scFv antibody by recombinant DNA technology and linked the scFv to the human IgG1 CH2CH3 (Fc). The resulting scFv-Fc represents the extracellular domain of the anti-FcµR CAR. The anti-FcμR scFv-Fc protein bound in a dose-dependent fashion to FcμR+ 293 cells but not to FcµR− 293 cells (Figure 2A). Cells of the leukemic cell line Mec-1 were also detected by the anti-FcμR scFv-Fc protein (Figure 2B) as were patients’ CLL cells (Figure 2B-C). Binding is specific because an scFv-Fc protein of irrelevant specificity did not bind to CLL cells. Although the anti-FcμR scFv-Fc protein recognized CLL cells, the same protein did not bind to CD19+ B cells and CD3+ T cells (Figure 2C). Data demonstrate that the anti-FcµR scFv-Fc antibody conserved the binding pattern of the parental 6B10 mAb.
The anti-FcμR scFv-Fc antibody conserves the binding specificity of the parental 6B10 antibody. (A) The anti-FcµR scFv-Fc antibody, which represents the extracellular CAR domain, specifically binds to FcµR+ cells. FcµR+ 293 cells (2 × 105 cells) were incubated in serial dilutions starting from 1000 ng/mL with anti-FcµR scFv-Fc. For control, 293 FcµR− cells were incubated with the anti-FcµR scFv-Fc protein (1000 ng/mL). Bound protein was detected by flow cytometry using the PE-conjugated anti-IgG antibody. Data represent the MFI of triplicates ± standard deviation (SD). (B) The anti-FcµR scFv-Fc binds specifically to Mec-1 and primary CLL cells. Mec-1 cells and patient’s CLL cells were incubated with the anti-FcµR scFv-Fc or, for control, with the anti-CEA scFv-Fc antibody of irrelevant specificity (each 1000 ng/mL). Bound scFv-Fc was detected by flow cytometry, and histograms were overlayed (light gray: anti-CEA scFv-Fc; dark gray: anti-FcµR scFv-Fc). (C) The anti-FcµR scFv-Fc retains binding specificity of the parental 6B10 mAb. CLL cells (n = 6) and B and T cells of healthy blood donors (n = 3 each) were incubated with anti-FcµR scFv-Fc or, for control, with the anti-CEA scFv-Fc (each 1000 ng/mL) and detected by the PE-conjugated anti-human IgG antibody (1:500). T cells and B cells were identified by anti-CD3 and anti-CD19 mAbs, and the MFI of bound scFv-Fc proteins was determined. Data represent mean values ± SD of the various donors. Statistical analysis was performed using the Student t test (**P < .01; ns, not significant).
The anti-FcμR scFv-Fc antibody conserves the binding specificity of the parental 6B10 antibody. (A) The anti-FcµR scFv-Fc antibody, which represents the extracellular CAR domain, specifically binds to FcµR+ cells. FcµR+ 293 cells (2 × 105 cells) were incubated in serial dilutions starting from 1000 ng/mL with anti-FcµR scFv-Fc. For control, 293 FcµR− cells were incubated with the anti-FcµR scFv-Fc protein (1000 ng/mL). Bound protein was detected by flow cytometry using the PE-conjugated anti-IgG antibody. Data represent the MFI of triplicates ± standard deviation (SD). (B) The anti-FcµR scFv-Fc binds specifically to Mec-1 and primary CLL cells. Mec-1 cells and patient’s CLL cells were incubated with the anti-FcµR scFv-Fc or, for control, with the anti-CEA scFv-Fc antibody of irrelevant specificity (each 1000 ng/mL). Bound scFv-Fc was detected by flow cytometry, and histograms were overlayed (light gray: anti-CEA scFv-Fc; dark gray: anti-FcµR scFv-Fc). (C) The anti-FcµR scFv-Fc retains binding specificity of the parental 6B10 mAb. CLL cells (n = 6) and B and T cells of healthy blood donors (n = 3 each) were incubated with anti-FcµR scFv-Fc or, for control, with the anti-CEA scFv-Fc (each 1000 ng/mL) and detected by the PE-conjugated anti-human IgG antibody (1:500). T cells and B cells were identified by anti-CD3 and anti-CD19 mAbs, and the MFI of bound scFv-Fc proteins was determined. Data represent mean values ± SD of the various donors. Statistical analysis was performed using the Student t test (**P < .01; ns, not significant).
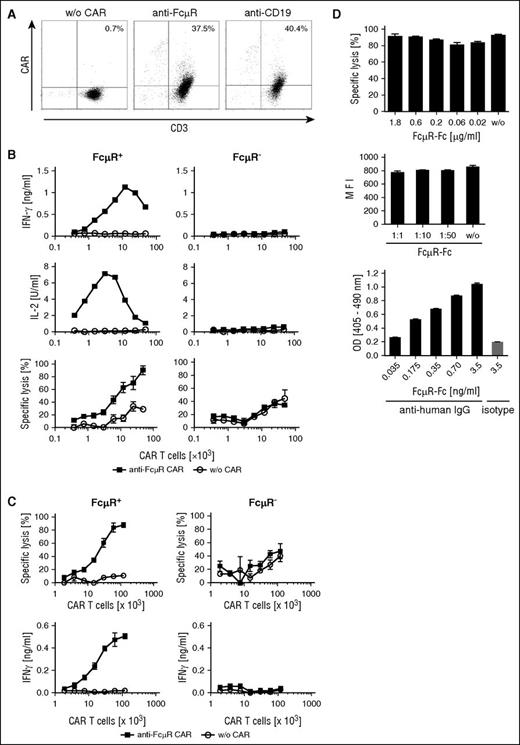
To redirect T cells specifically toward FcμR+ cells, we engineered a second-generation CAR with the 6B10 scFv-Fc domain for binding. The CAR contains the intracellular CD28 and CD3ζ signaling domain for T-cell activation. For comparison, a CD19-specific CAR of the same modular composition was used. As shown in Figure 3A, the anti-FcμR CAR was expressed on the surface of T cells as was the anti-CD19 CAR. The CAR levels on the surface of the modified T cells, as recorded by flow cytometry, were similar for both CARs.
The anti-FcµR CAR redirects T cells toward FcμR+ target cells even in the presence of soluble FcμR. (A) T cells of a healthy donor were genetically engineered with the CAR specific for FcμR and CD19, respectively. CAR expression by T cells was detected by flow cytometry using the PE-conjugated anti-IgG antibody, which binds to the CAR extracellular IgG1 domain and with the FITC-conjugated anti-CD3 antibody. Unmodified T cells (w/o CAR) were used as control. Numbers represent the percentage of T cells with CAR compared with the total number of T cells. One representative T-cell modification is shown. (B,C) T cells engineered with the anti-FcμR CAR were coincubated in increasing numbers with FcμR+ or FcμR− 293 cells (1.5 × 104 cells/well). Unmodified T cells (w/o CAR) served as control. Specific lysis of target cells was determined after 48 hours by the XTT-based viability assay (B) or after 4 hours by the calcein release assay (C). IFN-γ and IL-2 in the culture supernatants were determined by ELISA. Data represent the mean of triplicates ± SD. The assay was replicated 3 times. One representative assay is shown. (D) Specific lysis by CAR T cells was not blocked by soluble FcμR. FcµR+ 293 cells (1.5 × 104 cells) were coincubated with anti-FcµR CAR T cells in an effector-to-tumor cell ratio of 1.5:1 in the presence of serial dilutions of FcµR-Fc protein. Specific lysis of target cells (%) was determined by the XTT-based viability assay (upper chart). Data represent the mean of triplicates ± SD. Staining of FcµR+ 293 cells by the anti-FcµR mAb 6B10 was not blocked by the presence of serial dilutions of FcµR-Fc (start concentration: 3.5 µg/mL) (middle chart). Data represent the MFI of triplicates ± SD. As control, binding of the 6B10 mAb to FcµR-Fc was tested (lower chart). ELISA plates were coated with an anti-human IgG antibody or an isotype-matched control antibody (each 1 µg/mL). FcµR-Fc was incubated in serial dilutions, and bound protein was recorded by the anti-FcµR mAb 6B10 followed by a biotinylated anti-rat IgG, streptavidin-peroxidase, and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) as substrate. Data represent the mean of triplicates ± SD. The assay was replicated 3 times. One representative assay is shown. OD, optical density.
The anti-FcµR CAR redirects T cells toward FcμR+ target cells even in the presence of soluble FcμR. (A) T cells of a healthy donor were genetically engineered with the CAR specific for FcμR and CD19, respectively. CAR expression by T cells was detected by flow cytometry using the PE-conjugated anti-IgG antibody, which binds to the CAR extracellular IgG1 domain and with the FITC-conjugated anti-CD3 antibody. Unmodified T cells (w/o CAR) were used as control. Numbers represent the percentage of T cells with CAR compared with the total number of T cells. One representative T-cell modification is shown. (B,C) T cells engineered with the anti-FcμR CAR were coincubated in increasing numbers with FcμR+ or FcμR− 293 cells (1.5 × 104 cells/well). Unmodified T cells (w/o CAR) served as control. Specific lysis of target cells was determined after 48 hours by the XTT-based viability assay (B) or after 4 hours by the calcein release assay (C). IFN-γ and IL-2 in the culture supernatants were determined by ELISA. Data represent the mean of triplicates ± SD. The assay was replicated 3 times. One representative assay is shown. (D) Specific lysis by CAR T cells was not blocked by soluble FcμR. FcµR+ 293 cells (1.5 × 104 cells) were coincubated with anti-FcµR CAR T cells in an effector-to-tumor cell ratio of 1.5:1 in the presence of serial dilutions of FcµR-Fc protein. Specific lysis of target cells (%) was determined by the XTT-based viability assay (upper chart). Data represent the mean of triplicates ± SD. Staining of FcµR+ 293 cells by the anti-FcµR mAb 6B10 was not blocked by the presence of serial dilutions of FcµR-Fc (start concentration: 3.5 µg/mL) (middle chart). Data represent the MFI of triplicates ± SD. As control, binding of the 6B10 mAb to FcµR-Fc was tested (lower chart). ELISA plates were coated with an anti-human IgG antibody or an isotype-matched control antibody (each 1 µg/mL). FcµR-Fc was incubated in serial dilutions, and bound protein was recorded by the anti-FcµR mAb 6B10 followed by a biotinylated anti-rat IgG, streptavidin-peroxidase, and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) as substrate. Data represent the mean of triplicates ± SD. The assay was replicated 3 times. One representative assay is shown. OD, optical density.
To address whether the anti-FcμR CAR activates the engineered T cells, CAR T cells were incubated with FcμR+ and FcμR− 293 cells, and T-cell activation was recorded by monitoring IFN-γ and IL-2 released into the culture supernatants (Figure 3B-C). T cells with the anti-FcμR CAR secreted the cytokines in a dose-dependent fashion upon coincubation with FcμR+, but not with FcμR− 293 cells. T-cell activation was specific because unmodified T cells did not increase IL-2 or IFN-γ release. The anti-FcμR CAR T cells moreover exhibited specific cytolytic activity toward FcμR+ 293 cells, but not toward unmodified 293 cells. T cells without CAR did not lyse 293 cells with or without FcμR. Data obtained in the long- and short-term cytotoxicity assays (48 hours and 4 hours, respectively; Figure 3B vs C) were consistent.
Soluble FcµR accumulates in the serum of leukemia patients. We addressed the issue by adding FcµR-Fc in increasing concentrations to the in vitro assay for cytotoxicity. As shown in Figure 3D, specific lysis of FcµR+ 293 cells was not blocked by soluble FcµR-Fc in concentrations up to 1.8 μg/mL. Accordingly, staining of FcµR+ 293 cells by the 6B10 mAb was not blocked in the presence of FcµR-Fc. As control, the FcµR-Fc was recognized by the anti-FcµR mAb 6B10 as revealed by ELISA. Taken together, we conclude that the anti-FcµR CAR redirects and activates T cells in a specific fashion toward FcμR+ target cells even in the presence of soluble FcµR.
Anti-FcμR CAR T cells target patients’ CLL cells but not healthy B cells
We asked whether the anti-FcμR CAR sufficiently activates T cells to lyse the targeted leukemic cells. Cells of the leukemic line Mec-1 were used as target because the cells express FcμR and CD19 (Figure 4A). CAR-engineered T cells were coincubated in vitro with the Mec-1 cells, and target cell lysis was recorded. Mec-1 cells were eliminated by T cells engineered with the anti-FcμR CAR in a dose-dependent fashion. For comparison, T cells with the anti-CD19 CAR eliminated Mec-1 cells with similar efficiency. Lysis of Mec-1 cells was specific because T cells without CAR did not eliminate Mec-1 cells.
Anti-FcμR CAR T cells specifically lyse CLL cells but spare healthy B cells. (A) Mec-1 cells express FcμR and CD19 as revealed by flow cytometry after staining with the FITC-conjugated anti-FcμR mAb 6B10 or the PE-conjugated anti-CD19 antibody (bold line). Staining with an isotype-matched antibody served as control (gray histograms). T cells engineered with the anti-FcμR CAR and the anti-CD19 CAR, respectively, were coincubated in increasing numbers with FcμR+ CD19+ Mec-1 leukemia cells (1.5 × 104 cells/well). Unmodified T cells (w/o CAR) served as control. Specific lysis toward Mec-1 cells was determined after 48 hours by the XTT-based viability assay. (B) T cells from healthy donors were engineered with the anti-FcμR CAR and anti-CD19 CAR, respectively, or T cells without CAR (w/o) were co-incubated with patients’ CLL cells or healthy donor B cells (105 cells each) for 24 hours in an allogeneic setting (CLL cells, n = 11; B cells, n = 4). Alternatively, patients’ T cells were engineered with the respective CAR and incubated with the autologous CLL cells (n = 4). Engineered T cells of healthy donors were coincubated with the respective autologous B cells (n = 4). The CAR T-cell-to-target cell ratio was 5:1. CLL and healthy B cells were labeled with CFSE (0.83 μM) prior to coincubation to discriminate CLL and healthy B cells vs T cells. Staining of CLL and B cells for Annexin V was recorded by flow cytometry. Each dot represents an individual target cell donor. Statistic calculations were performed using the Student t test, *P < .05; ***P < .001; ns, not significant. (C) An example demonstrating killing of CLL cells by modified autologous CAR T cells. T cells from a CLL patient were modified with the respective CARs, and the CAR was recorded by flow cytometry using the PE-conjugated anti-IgG antibody, which binds to the CAR extracellular IgG1 domain and with the FITC-conjugated anti-CD3 antibody (left panel). Unmodified T cells (w/o CAR) were used as control. Numbers represent the percentage of T cells with CAR compared with the total number of T cells. T cells were cocultivated with autologous CLL cells in a CAR T-cell-to-CLL cell ratio of 5:1 for 24 hours, and the number of apoptotic CLL cells was determined by staining with APC-conjugated Annexin V (right panel). CLL cells were labeled with CFSE prior to coincubation to discriminate CLL cells from T cells. (D) CLL cells harbored an unmutated (n = 4) or mutated (n = 9) immunoglobulin heavy chain variable region (IgVH) status. Patients were in Binet stage A (n = 7), stage B (n = 3), or stage C (n = 5) (Table 2). Anti-FcµR CAR T cells showed an increased killing of CLL cells with an IgVH muted status compared with CLL cells with unmutated IgVH (P = .013). No significant differences in CAR T-cell killing of CLL cells were obtained from patients in different Binet stages. Each dot represents an individual CLL patient. Data represent the mean ± SD.
Anti-FcμR CAR T cells specifically lyse CLL cells but spare healthy B cells. (A) Mec-1 cells express FcμR and CD19 as revealed by flow cytometry after staining with the FITC-conjugated anti-FcμR mAb 6B10 or the PE-conjugated anti-CD19 antibody (bold line). Staining with an isotype-matched antibody served as control (gray histograms). T cells engineered with the anti-FcμR CAR and the anti-CD19 CAR, respectively, were coincubated in increasing numbers with FcμR+ CD19+ Mec-1 leukemia cells (1.5 × 104 cells/well). Unmodified T cells (w/o CAR) served as control. Specific lysis toward Mec-1 cells was determined after 48 hours by the XTT-based viability assay. (B) T cells from healthy donors were engineered with the anti-FcμR CAR and anti-CD19 CAR, respectively, or T cells without CAR (w/o) were co-incubated with patients’ CLL cells or healthy donor B cells (105 cells each) for 24 hours in an allogeneic setting (CLL cells, n = 11; B cells, n = 4). Alternatively, patients’ T cells were engineered with the respective CAR and incubated with the autologous CLL cells (n = 4). Engineered T cells of healthy donors were coincubated with the respective autologous B cells (n = 4). The CAR T-cell-to-target cell ratio was 5:1. CLL and healthy B cells were labeled with CFSE (0.83 μM) prior to coincubation to discriminate CLL and healthy B cells vs T cells. Staining of CLL and B cells for Annexin V was recorded by flow cytometry. Each dot represents an individual target cell donor. Statistic calculations were performed using the Student t test, *P < .05; ***P < .001; ns, not significant. (C) An example demonstrating killing of CLL cells by modified autologous CAR T cells. T cells from a CLL patient were modified with the respective CARs, and the CAR was recorded by flow cytometry using the PE-conjugated anti-IgG antibody, which binds to the CAR extracellular IgG1 domain and with the FITC-conjugated anti-CD3 antibody (left panel). Unmodified T cells (w/o CAR) were used as control. Numbers represent the percentage of T cells with CAR compared with the total number of T cells. T cells were cocultivated with autologous CLL cells in a CAR T-cell-to-CLL cell ratio of 5:1 for 24 hours, and the number of apoptotic CLL cells was determined by staining with APC-conjugated Annexin V (right panel). CLL cells were labeled with CFSE prior to coincubation to discriminate CLL cells from T cells. (D) CLL cells harbored an unmutated (n = 4) or mutated (n = 9) immunoglobulin heavy chain variable region (IgVH) status. Patients were in Binet stage A (n = 7), stage B (n = 3), or stage C (n = 5) (Table 2). Anti-FcµR CAR T cells showed an increased killing of CLL cells with an IgVH muted status compared with CLL cells with unmutated IgVH (P = .013). No significant differences in CAR T-cell killing of CLL cells were obtained from patients in different Binet stages. Each dot represents an individual CLL patient. Data represent the mean ± SD.
We explored whether CLL cells from the blood of leukemic patients were also eliminated by anti-FcμR CAR T cells. CLL cells were isolated, labeled with CFSE, and coincubated with CAR T cells from healthy donors. Anti-FcμR CAR T cells eliminated primary CLL cells from patients in various stages of the disease with high efficiency (Figure 4B). As expected, anti-CD19 CAR T cells eliminated CLL cells in a similar fashion. Of note, anti-FcμR CAR T cells did not eliminate healthy B cells from healthy donors, whereas the same T cells with the anti-CD19 CAR killed healthy B cells with similar efficiency as CD19+ CLL tumor cells.
We addressed whether patient’s T cells engineered with the anti-FcμR CAR also target autologous CLL cells. We isolated CLL cells and coincubated these cells with the autologous patient’s T cells engineered with the anti-FcμR or the anti-CD19 CAR, respectively. Anti-FcμR CAR T cells lysed autologous CLL cells as did the anti-CD19 CAR T cells (Figure 4B). Again, T cells with the anti-FcμR CAR did not eliminate autologous B cells of healthy donors, whereas anti-CD19 CAR did. Figure 4C exemplarily shows the killing of CLL cells by autologous T cells engineered with the anti-FcμR and the anti-CD19 CAR, respectively. CLL cells used in this study harbored a mutated or unmutated IgVH, and the patients were in different stages of the disease (Binet A, B, C) (Table 2). As summarized in Figure 4D, anti-FcμR CAR T cells eliminated CLL cells with mutated IgVH more efficiently than cells with unmutated IgVH; however, there was no difference in the in vitro killing efficacy dependent on the disease stage.
CLL patient characteristics
| Patient no. . | Age . | Sex . | IgVH mutational status . | Binet stage . |
|---|---|---|---|---|
| 1 | 56 | w | Mutated | B |
| 2 | 64 | m | Not mutated | C |
| 3 | 64 | m | Mutated | C |
| 4 | 71 | m | Mutated | A |
| 5 | 77 | m | Nonclassified | C |
| 6 | 43 | w | Mutated | A |
| 7 | 59 | w | Mutated | A |
| 8 | 49 | m | Mutated | A |
| 9 | 71 | m | Mutated | C |
| 10 | 69 | w | Mutated | A |
| 11 | 57 | w | Mutated | B |
| 12 | 53 | m | Not mutated | B |
| 13 | 64 | m | Not mutated | A |
| 14 | 59 | m | Not mutated | C |
| 15 | 61 | m | Nonclassified | A |
| Patient no. . | Age . | Sex . | IgVH mutational status . | Binet stage . |
|---|---|---|---|---|
| 1 | 56 | w | Mutated | B |
| 2 | 64 | m | Not mutated | C |
| 3 | 64 | m | Mutated | C |
| 4 | 71 | m | Mutated | A |
| 5 | 77 | m | Nonclassified | C |
| 6 | 43 | w | Mutated | A |
| 7 | 59 | w | Mutated | A |
| 8 | 49 | m | Mutated | A |
| 9 | 71 | m | Mutated | C |
| 10 | 69 | w | Mutated | A |
| 11 | 57 | w | Mutated | B |
| 12 | 53 | m | Not mutated | B |
| 13 | 64 | m | Not mutated | A |
| 14 | 59 | m | Not mutated | C |
| 15 | 61 | m | Nonclassified | A |
CAR T cells secreted increasing amounts of the proinflammatory cytokines IFN-γ and TNF-α upon coincubation with autologous CLL cells as revealed by a bead-based multiplex assay (Figure 5). Cytokine release increased to a similar extent upon activation of anti-FcμR and anti-CD19 CAR T cells. In accordance with the cytolytic activity, the release of proteins associated with cytolysis like soluble FasL and granzyme B was increased by both anti-FcμR and anti-CD19 CAR T cells compared with T cells without CAR.
Anti-FcμR CAR T cells secrete a panel of cytokines and lytic proteins after coincubation with CLL cells. Patients’ T cells with anti-FcμR CAR, anti-CD19 CAR, or without CAR (w/o), respectively, were coincubated with autologous CLL cells (105 cells) for 24 hours (n = 4) in a CAR T-cell-to-CLL cell ratio of 5:1. CLL cells without added T cells served as additional control. The various proteins in the culture supernatant were detected using the bead-based “LEGENDplex multi-analyte assay.”
Anti-FcμR CAR T cells secrete a panel of cytokines and lytic proteins after coincubation with CLL cells. Patients’ T cells with anti-FcμR CAR, anti-CD19 CAR, or without CAR (w/o), respectively, were coincubated with autologous CLL cells (105 cells) for 24 hours (n = 4) in a CAR T-cell-to-CLL cell ratio of 5:1. CLL cells without added T cells served as additional control. The various proteins in the culture supernatant were detected using the bead-based “LEGENDplex multi-analyte assay.”
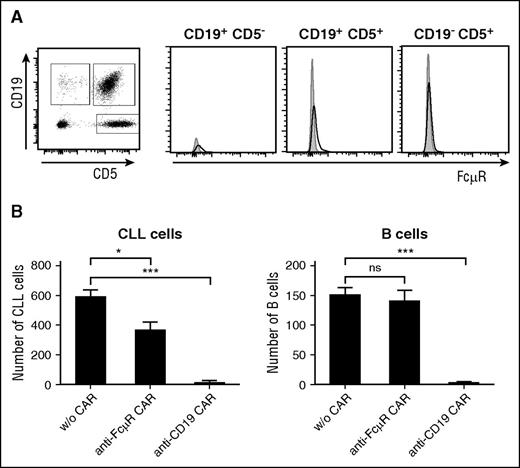
We addressed whether healthy B cells from CLL patients were spared by anti-FcμR CAR T cells while attacking the CLL cells in the same sample. The CD19+ CD5+ CLL cells, CD19+ CD5− B cells, and CD19−CD5+ T cells were identified by flow cytometry, and the levels of FcμR protein in the individual cell subsets were recorded (Figure 6A). The heterogeneous cell population was coincubated with T cells engineered with anti-FcμR CAR or anti-CD19 CAR, respectively. Anti-FcμR CAR T cells eliminated CLL cells, as did anti-CD19 CAR T cells; however, the healthy B cells were not eliminated by the anti-FcμR CAR T cells, but by the anti-CD19 CAR T cells (Figure 6B). The data again demonstrate the superior selectivity of anti-FcμR CAR T cells in eliminating CLL cells while sparing healthy B cells, whereas anti-CD19 CAR T cells eliminate both CLL and healthy B cells.
Selective targeting of patient’s CLL cells in the presence of healthy B cells by anti-FcµR CAR T cells. (A) Peripheral blood lymphocytes (0.5 × 106 cells) from a CLL patient were stained with the APC-conjugated anti-CD5 mAb and the PE-conjugated anti-CD19 mAb. The cell populations (CD19+ CD5− non-CLL B cells, CD19−CD5+ T cells, CD19+ CD5+ CLL cells) were recorded for FcµR using the FITC-conjugated anti-FcµR mAb 6B10 (black line). An isotype-matched control antibody served as control (gray histogram). Histograms exemplarily show FcµR levels of each subpopulation. (B) T cells with and without anti-FcμR CAR and anti-CD19 CAR, respectively, were coincubated with PBMCs from a CLL patient (105 cells) in an effector-to-CLL cell ratio of 5:1 for 24 hours. Cells were stained with the FITC-conjugated anti-CD5 mAb and the PE-conjugated anti-CD19 mAb. The number of CD19+ CD5+ CLL cells and CD19+ CD5− healthy B cells in the same patient sample was recorded by flow cytometry. Bars represent the cell numbers; data represent the mean of triplicates ± SD. Statistical analysis was performed using the Student t test (*P < .05; ***P < .001; ns, not significant).
Selective targeting of patient’s CLL cells in the presence of healthy B cells by anti-FcµR CAR T cells. (A) Peripheral blood lymphocytes (0.5 × 106 cells) from a CLL patient were stained with the APC-conjugated anti-CD5 mAb and the PE-conjugated anti-CD19 mAb. The cell populations (CD19+ CD5− non-CLL B cells, CD19−CD5+ T cells, CD19+ CD5+ CLL cells) were recorded for FcµR using the FITC-conjugated anti-FcµR mAb 6B10 (black line). An isotype-matched control antibody served as control (gray histogram). Histograms exemplarily show FcµR levels of each subpopulation. (B) T cells with and without anti-FcμR CAR and anti-CD19 CAR, respectively, were coincubated with PBMCs from a CLL patient (105 cells) in an effector-to-CLL cell ratio of 5:1 for 24 hours. Cells were stained with the FITC-conjugated anti-CD5 mAb and the PE-conjugated anti-CD19 mAb. The number of CD19+ CD5+ CLL cells and CD19+ CD5− healthy B cells in the same patient sample was recorded by flow cytometry. Bars represent the cell numbers; data represent the mean of triplicates ± SD. Statistical analysis was performed using the Student t test (*P < .05; ***P < .001; ns, not significant).
We recorded the therapeutic efficacy of anti-FcμR CAR T cells in comparison with anti-CD19 CAR T cells in a murine xenograft model of human CLL.20 Systemically applied leukemic Mec-1 cells infiltrate multiple organs and extensively amplify in the immune-deficient mice causing dysfunction of the motoric nerves as an early disease symptom. The onset of Mec-1-caused disease was significantly delayed by applying anti-FcμR CAR T cells compared with treatment without T cells (P < .01); the same effect was observed upon application of anti-CD19 CAR T cells (Figure 7). We conclude that T cells with the anti-FcμR CAR exhibited therapeutic efficacy in eliminating Mec-1 cells in a mouse xenograft model as did the anti-CD19 CAR T cells.
Anti-FcμR and anti-CD19 CAR T cells suppress growth of Mec-1 cells with similar efficiency in a xenogenic mouse model. Mec-1 cells (5 × 106/mouse) were intravenously coinjected with human CAR T cells (3 × 106/mouse) or without T cells for control into Rag2−/− cγ−/− mice (n = 7 per group). Mice were inspected every 1 to 3 days for neurological symptoms due to outgrowth of injected Mec-1 cells. Disease-free survival was recorded; data were presented in a Kaplan-Meier plot (anti-FcμR CAR T cells vs w/o T cells, P < .01; anti-CD19 CAR T cells vs w/o T cells, P < .01 as determined by the Log-rank test).
Anti-FcμR and anti-CD19 CAR T cells suppress growth of Mec-1 cells with similar efficiency in a xenogenic mouse model. Mec-1 cells (5 × 106/mouse) were intravenously coinjected with human CAR T cells (3 × 106/mouse) or without T cells for control into Rag2−/− cγ−/− mice (n = 7 per group). Mice were inspected every 1 to 3 days for neurological symptoms due to outgrowth of injected Mec-1 cells. Disease-free survival was recorded; data were presented in a Kaplan-Meier plot (anti-FcμR CAR T cells vs w/o T cells, P < .01; anti-CD19 CAR T cells vs w/o T cells, P < .01 as determined by the Log-rank test).
Discussion
Targeting of CLL cells with anti-CD19 CAR T cells produces remarkable remission in current trials; however, the “on-target off-tumor” autoimmunity with lasting B-cell aplasia demands for alternative antigens suitable for more selective T-cell targeting toward CLL cells. We revealed FcμR as a potential target antigen with a beneficial target profile for a CAR T-cell attack based on the following properties. FcμR is highly expressed by nearly all CLL cells but far less by healthy B cells, T cells, and natural killer cells, whereas other blood cell types such as myeloid cells and erythrocytes do not express FcμR (compare Figure 1).13,21,22 For detecting FcμR, we used the 6B10 mAb,12 which has a similar detection profile as the other tested anti-FcμR antibodies, including FAIM3, FAIM3 1E4, and HM14, however, it recognizes a different FcμR epitope.
In order to achieve selective CLL targeting, the expression profile of FcμR, that is, high expression by CLL cells vs low expression by healthy lymphocytes, is a significant advantage compared with CD19, which is higher expressed by healthy B cells than by CLL cells and currently used as target. FcμR is furthermore a good target because the receptor is involved in the pathogenesis of CLL and in the progression of the disease by concomitant B-cell receptor and Toll-like receptor activation, thereby sustaining the survival of the leukemic cells.12
For engineering the CAR, we converted the rat monoclonal anti-FcμR antibody 6B10 into an scFv, which retained the binding specificity for FcμR. The scFv was used as binding domain for the CAR and shown to selectively bind to CLL cells and not to B and T cells. The engineered CAR harbors the combined CD28-CD3ζ signaling chain in the intracellular moiety to provide the primary and the costimulatory signal for T-cell activation upon FcμR binding. The anti-FcμR CAR efficiently redirected T cells toward CLL cells and mediated full T-cell activation upon antigen engagement indicated by the release of pro-inflammatory cytokines like IFN-γ, IL-2, and TNF-α, of cytolytic proteins like soluble FasL and granzyme B, and, finally, by specific lysis of FcμR+ cells. Established Mec-1 leukemic cells and isolated leukemic cells from CLL patients were recognized and efficiently eliminated by anti-FcμR CAR T cells, whereas cells lacking FcμR were not attacked, demonstrating the antigen specificity of the redirected CAR T-cell attack. Moreover, antileukemic cell activity was also recorded when redirecting patients’ anti-FcμR CAR T cells toward their autologous CLL cells. The observation is of major significance because peripheral blood T cells from patients with cancer are frequently anergic and in a terminally differentiated and exhausted state.23
However, healthy B cells were not substantially eliminated by the anti-FcμR CAR T cells, which is in contrast to anti-CD19 CAR T cells. We think this is notable because some B cells showed minor FcμR levels on the cell surface, implying that these FcμR levels were below threshold to initiate a CAR-mediated T-cell activation. The situation is in contrast to the high levels of CD19 on healthy B cells, often higher than on CLL cells, which always produced a full-blown T-cell response against these cells resulting in lasting B-cell aplasia in nearly all successfully treated patients. Taken together, the beneficial properties of FcμR as target and the highly specific binding domain make the anti-FcμR CAR T-cell therapy more selective for CLL cells with respect to sparing healthy B cells compared with the currently used anti-CD19 CAR T cells.
Compared with healthy donors, CLL patients often have high serum titers of soluble FcμR, which is an alternatively spliced form of the receptor; serum FcμR correlates with the number of circulating CLL cells.24 In vitro, soluble FcμR did not block CAR T-cell–mediated killing of FcμR+ cells (compare Figure 3D), implying that the anti-FcμR CAR T-cell therapy will also be efficacious in patients with high serum FcμR levels. The data are in accordance with other reports that soluble ligands do not substantially block CAR redirected T-cell activation, for example, serum CEA25,26 or serum CD30.27,28
The modular CAR design allows a broad range of further routine optimizations, for instance, using a modified CD28 signaling domain29 or other costimulatory domains like 4-1BB or spacer moieties of different lengths,30,31 in order to fine tune the T-cell response. Instead of the anti-FcμR scFv for binding, the IgM Cμ2-Cμ3-Cμ4 constant domain, which is the natural ligand for FcμR, may be used as described for an anti-FcμR immune toxin, which showed preferential elimination of CLL cells.32 For the treatment of CLL, however, we think that targeting by an antibody is more selective because the IgM constant domain also binds to the polymeric immunoglobulin receptor and the FCAMR (Fcα/μ receptor),21 which would diminish the selectivity in CAR targeting. Alternatively, ROR-1 was also proposed for CAR T-cell targeting of CLL cells.33 Like FcμR, ROR-1 shows preferential and uniform expression on the surface of CLL cells and less on healthy lymphocytes. However, ROR-1 is also expressed by mature adipocytes bearing some risk of some “on-target off-tumor” toxicities to adipose tissues in treated patients, although no toxicity was observed in macaques.34 In this situation, FcμR may show a more restricted and thereby a more favorite expression pattern for a CLL-specific CAR T-cell therapy.
Preclinical models and clinical observations strongly suggest that the therapeutic effect of CAR T-cell therapy requires engraftment and amplification of modified T cells after administration. Reevaluation of treated patients revealed circulating anti-CD19 CAR T cells even more than 3 years after application.35 Repetitive restimulation of the anti-CD19 CAR T cells by the emerging healthy B cells may help to prolong the T-cell survival through antiapoptotic signaling, thereby sustaining a pool of viable, restimulated CAR T cells large enough to control the disease. Such ongoing restimulation of CAR T cells by targeting healthy B cells may contribute to the long-lasting control of the disease. This mechanism will not apply to anti-FcμR CAR T cells; whether lack of such restimulation through healthy cells impacts therapeutic efficacy needs to be explored in clinical trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Petra Hofmann and Nicole Riet for excellent technical support and Gunter Rappl, Central Cell Sorting Facility, Center for Molecular Medicine Cologne, Cologne, Germany, for technical assistance in cell sorting.
This study was supported by a grant from the Deutsche José Carreras–Leukämie Stiftung, München, Germany (H.A.), and the CLL Global Research Foundation, Houston, Texas (C.-M.W.).
Authorship
Contribution: E.F., A.A.H., L.P.F., C.-M.W., and H.A. conceived and designed the work; E.F. performed the experimental analyses; E.F., A.A.H., and H.A. analyzed the data; and E.F., A.A.H., C.-M.W., and H.A. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hinrich Abken, Center for Molecular Medicine Cologne, University of Cologne, Robert-Koch-Str 21, D-50931 Cologne, Germany; e-mail: hinrich.abken@uk-koeln.de.