Key Points
Reversible oxidation of GAPDH promotes metabolic reprogramming of stored RBCs, as gleaned through tracing with 13C1,2,3-glucose.
Storage-induced redox imbalance promotes vesiculation of irreversibly oxidized GAPDH, as determined through switch-tag redox proteomics.
Abstract
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) plays a key regulatory function in glucose oxidation by mediating fluxes through glycolysis or the pentose phosphate pathway (PPP) in an oxidative stress–dependent fashion. Previous studies documented metabolic reprogramming in stored red blood cells (RBCs) and oxidation of GAPDH at functional residues upon exposure to pro-oxidants diamide and H2O2. Here we hypothesize that routine storage of erythrocyte concentrates promotes metabolic modulation of stored RBCs by targeting functional thiol residues of GAPDH. Progressive increases in PPP/glycolysis ratios were determined via metabolic flux analysis after spiking 13C1,2,3-glucose in erythrocyte concentrates stored in Additive Solution-3 under blood bank conditions for up to 42 days. Proteomics analyses revealed a storage-dependent oxidation of GAPDH at functional Cys152, 156, 247, and His179. Activity loss by oxidation occurred with increasing storage duration and was progressively irreversible. Irreversibly oxidized GAPDH accumulated in stored erythrocyte membranes and supernatants through storage day 42. By combining state-of-the-art ultra-high-pressure liquid chromatography–mass spectrometry metabolic flux analysis with redox and switch-tag proteomics, we identify for the first time ex vivo functionally relevant reversible and irreversible (sulfinic acid; Cys to dehydroalanine) oxidations of GAPDH without exogenous supplementation of excess pro-oxidant compounds in clinically relevant blood products. Oxidative and metabolic lesions, exacerbated by storage under hyperoxic conditions, were ameliorated by hypoxic storage. Storage-dependent reversible oxidation of GAPDH represents a mechanistic adaptation in stored erythrocytes to promote PPP activation and generate reducing equivalents. Removal of irreversibly oxidized, functionally compromised GAPDH identifies enhanced vesiculation as a self-protective mechanism in ex vivo aging erythrocytes.
Introduction
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is an evolutionarily conserved enzyme that controls glucose flux through the canonical Embden-Meyerhof glycolytic pathway.1 Under pro-oxidant conditions, GAPDH contributes to a glycolytic bottleneck that favors a metabolic switch toward the pentose phosphate pathway (PPP).1 The mechanisms underlying this redox-dependent metabolic adaptation through GAPDH involve its functional active site pocket. Indeed, the GAPDH catalytic site is characterized by redox-sensitive amino acid residues, Cys152 and His179, which modulate the metabolic activity of GAPDH in an oxidative stress–dependent fashion.2 These residues are hydrogen bonding partners, promoting the thiolate form of Cys152 required for catalysis. Other redox-sensitive residues, such as surface-exposed Cys247, mediate GAPDH multimerization and contribute to noncanonical functions.2 For example, it recently emerged that oxidized/extracellular GAPDH can play nonglycolytic moonlighting functions, such as participating in iron homeostasis by scavenging transferrin-bound iron in circulating blood cells.3
Though these mechanisms have been largely appreciated in vitro, following the exogenous addition of pro-oxidants such as diamide and H2O2 at elevated concentrations (eg, 5 mM),2,4 to the best of our knowledge no direct mass spectrometry (MS)-based evidence of irreversible GAPDH oxidation at functional residues in absence of excess of pro-oxidants either in vivo or ex vivo has been reported thus far.
Red blood cell (RBC) storage in the blood bank is a life-saving therapeutic intervention for ∼5 million Americans every year.5 Packed RBCs are routinely stored at ∼4°C under sterile blood bank conditions for up to 42 days. However, routine storage is associated with the progressive accumulation of a long series of biochemical alterations to stored erythrocytes, collectively referred to as the “storage lesion.”5 Though the clinical relevance of such lesions is not fully clear, biochemical evidence suggests that RBCs stored longer than 2 weeks are energetically and oxidatively challenged and thus potentially less functional than fresh RBCs (for a comprehensive review, see D’Alessandro et al5 ). Recently, it has been argued that metabolic reprogramming might underlie the onset and progression of the storage lesion.6 Impairments of energy and redox homeostasis are proposed to trigger alterations to structural lipids and proteins, thereby promoting the progressive accumulation of morphological changes and thus reducing RBC deformability and survival upon transfusion. Borrowing from in vitro experiments of RBC metabolic adaptations upon exposure to pro-oxidant environments,7 proposed models suggest a role for redox-sensitive glycolytic enzymes, GAPDH above all, in mediating the so-called oxygen-dependent metabolic modulation.8 This model posits that oxygen saturation levels modulate the competitive binding of glycolytic enzymes and deoxyhemoglobin with the N-terminal cytosolic domain of band 3.7 Under pro-oxidant conditions, hemoglobin (Hb) becomes oxygenated, decreasing its affinity for band 3.7 In turn, glycolytic enzymes, including GAPDH, migrate to the cytosolic domain of band 3 or alternative binding sites on the same or nearby structural proteins.7 Relocation of GAPDH to the membrane and binding to band 3 have been associated with loss of activity, promoting a metabolic shift from glycolysis to the PPP, fueling the generation of the reducing equivalent reduced nicotinamide adenine dinucleotide phosphate (NADPH) to preserve glutathione homeostasis.7 However, such regulatory mechanisms are progressively lost during routine storage of nonleukocyte-filtered erythrocyte concentrates stored in saline adenine glucose mannitol (SAGM) additives in the blood bank,5 though similar evidence is missing for leukocyte-filtered packed RBCs stored in more common additives for the United States (eg, Additive Solution-3 [AS-3]). This consideration is relevant in light of the role of additive solutions other than SAGM, in particular AS-3,9-11 and leukofiltration12,13 in reducing the storage lesion, as previously reported. Recently, it has been suggested that the partial loss of metabolic modulation of SAGM RBCs8,14 may be in part due to the oxidation of redox-sensitive thiols in cytosolic GAPDH, leading to enzyme inactivation.15 Recently, Rinalducci and colleagues elegantly showed that cytosolic GAPDH in leukocyte-filtered SAGM erythrocytes is reversibly oxidized at Cys152 and 156 through the generation of an intramolecular disulfide bridge.15 However, no irreversible oxidative modifications of potentially active7 membrane-bound GAPDH were documented.
Here we exploit a combination of state-of-the-art quantitative metabolic flux analysis,16 structural modeling, targeted absolute protein quantification via QconCAT technology,17 and a switch-tag redox proteomics approach18 to investigate the role of GAPDH oxidation in metabolic reprogramming of stored RBCs. By combining these technologies with RBC storage under hyperoxic and hypoxic conditions (SO2 >90% and <5%, respectively), our study provides the first evidence of a GAPDH-dependent redox-regulatory mechanism of metabolism in stored RBCs.
Methods
For more detailed protocols, see supplemental Methods (available on the Blood Web site). Commercial reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Sample collection
Blood was collected from healthy donors in accordance with the Declaration of Helsinki, and log4 leukocyte-filtered (Pall Medical, Braintree, MA) packed RBCs were stored in CP2D-AS-3 (n = 5, Haemonetics Corp, Braintree, MA). Units were sterilely sampled (15 mL per time point) at days 0, 21, 42, and cells and supernatants were sorted through centrifugation at 2000g for 10 minutes at 4°C at storage day 42. Subfractionation to obtain membranes and vesicles is detailed in supplemental Methods.
Metabolic labeling experiments
A set of packed RBCs (n = 4) was collected, processed, and stored in CP2D-AS-3, as described in “Sample collection.” Before processing, AS-3 (containing 55 mM glucose) was supplemented with 11 mM 13C1,2,3-glucose (Cambridge Isotope Laboratories, Tewksbury, MA). Samples collected at storage days 2, 7, 14, 21, 28, 35, and 42, and cells and supernatants were sorted and extracted as detailed in the supplemental Methods.
2,3-DPG assay
Levels of 2,3-diphosphoglcyerate (2,3-DPG) were determined through the UV test for the determination of 2,3-DPG in the blood research samples kit (Roche, Mannheim, Germany).
UHPLC-MS metabolomics
RBCs and supernatants were processed as previously described9 and analyzed via ultra-high-pressure liquid chromatography–mass spectrometry (UHPLC-MS) (Vanquish, Q Exactive; Thermo Fisher, San Jose, CA and Bremen, Germany). Metabolite assignments and isotopologue distributions were determined against internal standard libraries through Maven.19,20 Technical reproducibility (coefficients of variation) was assessed by monitoring heavy labeled standard mixes and the xenometabolite 5-fluorouracil (2.5 µM). Absolute quantitation was performed against heavy standards spiked into each sample at known concentrations.
GAPDH activity assay
GAPDH activity was assessed in total RBCs and supernatants at storage days 2, 21, and 42 using a commercial GAPDH activity assay kit (BioVision, Inc, Milpitas, CA). All stock solutions were freshly prepared. Aliquots of total RBCs and their supernatants were diluted 1 to 20 and 1 to 2, respectively, then 5 μL of each was used. The assay was performed in a 96-well plate (final volume = 100 μL per well) in technical triplicate at 37°C. Reversible oxidation was assessed following the addition of dithiothreitol (DTT) at time zero (2.5 mM). The absorbance at 450 nm was recorded each minute for 45 minutes.
In-gel digestion with MS/MS
Proteomic analyses on RBC membranes and vesicles were performed as previously reported using 30 µg of protein per time point (days 0, 21, 42 membranes and day 42 vesicles) and a 4% to 12% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel.21 Bands were reduced, alkylated, and trypsin digested, then analyzed by nanoscale liquid chromatography (nanoLC)-MS/MS (Eksigent nanoLC and Thermo Orbitrap Velos Pro), as detailed in the supplemental Methods.
Switch-tagging redox proteomics
Albumin and immunoglobulin G (IgG) were removed from 600 µg of RBC supernatants per time point using serum protein immunodepletion resins (Proteome Purify2; R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. After incubation, the resin was transferred to a 0.22-µm filter unit (Spin-X) and centrifuged for 2 minutes at 2000g. Depleted supernatants were concentrated (3000 molecular weight cutoff [MWCO]; EMD Millipore, Billerica, MA), normalized, then incubated with N-ethylmaleimide (NEM; 100 mM) for 15 minutes. RBCs were lysed in 9 parts of cold water and incubated on ice for 5 minutes, then 30 µL of the samples were treated with additional NEM for 15 minutes.
Proteins were separated using SDS-PAGE, and 5 bands were excised per lane in the range of ∼20 to 80 kDa and digested as described in the supplemental Methods. Extracted peptides were analyzed by nanoLC-MS/MS (Thermo EASY-nLC 1000 and Q Exactive HF). Peptides were separated on a house-made 15-cm C18 analytical column (100 µm inner diameter) packed with Cortecs C18 resin (2.7 µm; Phenomenex). Peptides were separated by a 80-minute linear gradient of 2% to 32% acetonitrile (ACN) at 350 nL per minute.
QconCAT-based absolute quantitation of GAPDH in supernatants
QconCAT constructs were designed to quantify GAPDH in RBC supernatants at storage days 0, 21, and 4217 using the following peptide: LISWYDNEFGYSNR. QconCAT proteins were expressed and purified as previously described.22 Known concentrations of QconCAT proteins were mixed with depleted RBC supernatants, and resulting samples were reduced, alkylated, and digested using a filter-aided sample preparation protocol as previously described.22
Targeted selected reaction monitoring (SRM) quantification was performed using a QTRAP 5500 (SCIEX, Framingham, MA) interfaced with a Dionex Ultimate 3000 UHPLC system (Thermo). Five micrograms of peptides was injected for each sample and loaded onto an Acquity BEH C18 column (1.7 µm, 150 × 1 mm; Waters, Milford, MA) with 5% ACN at 30 µL per minute for 3 minutes. Peptides were separated using a gradient of 5% to 40% ACN over 30 minutes.
Structural models
SO2 measurements and MetHb concentration
Preparation of controlled >95% (hyperoxic) or 5% (hypoxic) SO2 of day 0 CP2D-AS-3 RBCs in vented chambers (Difco BLL, Detroit, MI) and determination of SO2 and MetHb percentages were performed as previously reported.24
Results
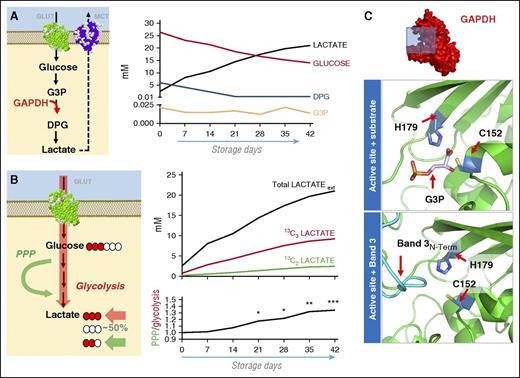
Metabolomics analysis of stored RBCs revealed a progressive decrease of extracellular glucose and increase in lactate, suggestive of ongoing glycolysis (Figure 1A). However, consumption of GAPDH substrate glyceraldehyde 3-phosphate and generation of GAPDH product diphosphoglycerate decreased significantly after 7 and 14 days in comparison with day 1 controls under routine storage conditions (Figure 1A). Metabolic flux analysis was determined by quantitation of 13C3 and 13C2 isotopologues of lactate, either derived from catabolism of 13C1,2,3-glucose via glycolysis or PPP, respectively (Figure 1B). Results indicate that lactate accumulation was dependent on ongoing glycolysis and activation of the PPP after storage day 7, which became statistically significant by storage day 21 (Figure 1B).
Oxidative modulation of glucose metabolism during RBC storage. (A) Metabolic analysis of glycolytic intermediates in AS-3 RBCs from storage day 1 to storage day 42. Despite ongoing glycolysis, as illustrated by lactate accumulation, glyceraldehyde 3-phosphate and diphosphoglycerate reach steady-state levels by storage days 14-21. (B) Metabolic flux analysis was performed by spiking 11 mM 13C1,2,3-glucose (in addition to the 55 mM dextrose in AS-3 formula) into RBC concentrates at day 0. The isotopologues +3 or +2 of lactate accumulate proportionally to glucose catabolic fluxes through the Embden-Meyerhof glycolytic pathway or through the PPP, respectively. The first carbon atom of glucose is lost in the form of CO2 during metabolism through the oxidative phase of the PPP. Metabolic fluxes were determined in lactate, and absolute quantitation of total lactate and isotopologues was performed directly against spiked in 13C1-lactate (upon correction for 3.3% natural abundance) and indirectly by determining the percentage of labeled lactate in total lactate, quantified through classic spectrometric approaches. PPP to glycolysis ratios were determined by dividing lactate isotopologues (+2/+3) at each tested time point (2-42, on a weekly basis). (C) An overview of the GAPDH monomer structure (pdb ID: 3GPD) is shown. Structural models of the enzyme active site pocket, highlighting Cys152 and His179 are shown in presence of the substrate glyceraldehyde 3-phosphate or the N-term cytosolic domain of band 3 (PDB ID:3BTB), in agreement with Eisenmesser and Post.23
Oxidative modulation of glucose metabolism during RBC storage. (A) Metabolic analysis of glycolytic intermediates in AS-3 RBCs from storage day 1 to storage day 42. Despite ongoing glycolysis, as illustrated by lactate accumulation, glyceraldehyde 3-phosphate and diphosphoglycerate reach steady-state levels by storage days 14-21. (B) Metabolic flux analysis was performed by spiking 11 mM 13C1,2,3-glucose (in addition to the 55 mM dextrose in AS-3 formula) into RBC concentrates at day 0. The isotopologues +3 or +2 of lactate accumulate proportionally to glucose catabolic fluxes through the Embden-Meyerhof glycolytic pathway or through the PPP, respectively. The first carbon atom of glucose is lost in the form of CO2 during metabolism through the oxidative phase of the PPP. Metabolic fluxes were determined in lactate, and absolute quantitation of total lactate and isotopologues was performed directly against spiked in 13C1-lactate (upon correction for 3.3% natural abundance) and indirectly by determining the percentage of labeled lactate in total lactate, quantified through classic spectrometric approaches. PPP to glycolysis ratios were determined by dividing lactate isotopologues (+2/+3) at each tested time point (2-42, on a weekly basis). (C) An overview of the GAPDH monomer structure (pdb ID: 3GPD) is shown. Structural models of the enzyme active site pocket, highlighting Cys152 and His179 are shown in presence of the substrate glyceraldehyde 3-phosphate or the N-term cytosolic domain of band 3 (PDB ID:3BTB), in agreement with Eisenmesser and Post.23
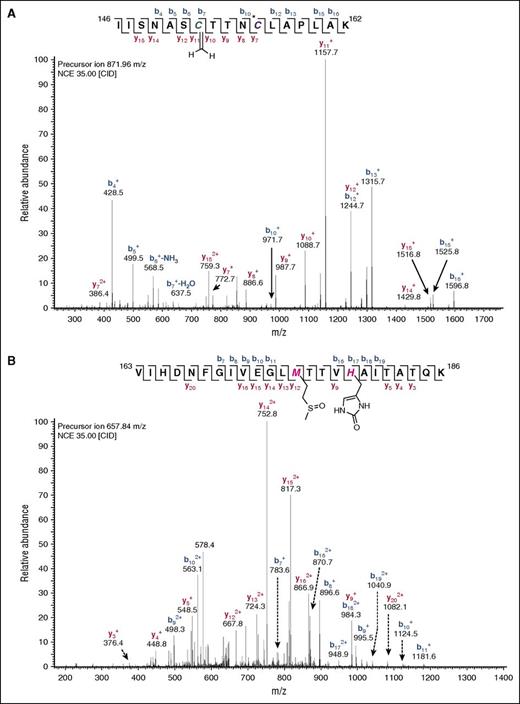
Oxidation of active site residues Cys152 and His179 (Figure 1C) would compromise membrane binding and catalytic activity of GAPDH. During storage, especially at day 42, redox-sensitive residues Cys152, 156, and 247, and His165 and 179, in the membrane-bound and vesicle fractions were observed to be oxidatively modified, as judged through the identification of mass additions and fragmentation spectra consistent with Cys-SO2H and 2-oxo-His (Table 1). In addition, cysteine to dehydroalanine (DHA) conversion was observed for all 3 functional cysteines (Table 1). Two representative MS2 spectra for Cys152 and His179 oxidation in day 42 RBC membrane-bound GAPDH are shown in Figure 2, panels A and B, respectively. The loss of the thiol nucleophile through elimination to DHA at Cys152 and oxidation of His179 are expected to irreversibly compromise residual membrane-bound GAPDH activity (Figure 3A).
GAPDH Cys and His modifications in stored RBC membrane and vesicle
| . | C152* . | C156* . | C247* . | H165* . | H179* . | m/z [Theor.] . | m/z [Exp.] . | Δ ppm . | Score† . |
|---|---|---|---|---|---|---|---|---|---|
| IISNASCTTNCLAPLAK | CM | CM | 917.4640 | 917.4630 | 1.1 | 108.2 | |||
| Diox. | CM | 904.9482 | 904.9445 | 4.1 | 33.5 | ||||
| CM | Diox. | 904.9482 | 904.9448 | 3.8 | 40.9 | ||||
| DHA | CM | 871.9595 | 871.9570 | 2.9 | 86.0 | ||||
| CM | DHA | 871.9595 | 871.9559 | 4.1 | 52.9 | ||||
| Diox. | DHA | 859.4436 | 859.4316 | 13.9 | 53.9 | ||||
| DHA | Diox. | 859.4436 | 859.4318 | 13.7 | 51.5 | ||||
| Sulfur dioxide | CM | 920.9265 | 920.9309 | 4.8 | 91.5 | ||||
| VPTANVSVVDLTCR | CM | 765.9014 | 765.9017 | 0.4 | 92.7 | ||||
| Diox. | 753.3856 | 753.3828 | 3.7 | 60.2 | |||||
| DHA | 720.3968 | 720.3950 | 2.5 | 68.1 | |||||
| VIHDNFGIVEGLMTTVHAITATQK | Oxidation | Unmodified | 871.1159 | 871.1215 | 6.4 | 57.7 | |||
| Unmodified | Oxidation | 876.4475‡ | 876.4535 | 6.8 | 51.2 |
| . | C152* . | C156* . | C247* . | H165* . | H179* . | m/z [Theor.] . | m/z [Exp.] . | Δ ppm . | Score† . |
|---|---|---|---|---|---|---|---|---|---|
| IISNASCTTNCLAPLAK | CM | CM | 917.4640 | 917.4630 | 1.1 | 108.2 | |||
| Diox. | CM | 904.9482 | 904.9445 | 4.1 | 33.5 | ||||
| CM | Diox. | 904.9482 | 904.9448 | 3.8 | 40.9 | ||||
| DHA | CM | 871.9595 | 871.9570 | 2.9 | 86.0 | ||||
| CM | DHA | 871.9595 | 871.9559 | 4.1 | 52.9 | ||||
| Diox. | DHA | 859.4436 | 859.4316 | 13.9 | 53.9 | ||||
| DHA | Diox. | 859.4436 | 859.4318 | 13.7 | 51.5 | ||||
| Sulfur dioxide | CM | 920.9265 | 920.9309 | 4.8 | 91.5 | ||||
| VPTANVSVVDLTCR | CM | 765.9014 | 765.9017 | 0.4 | 92.7 | ||||
| Diox. | 753.3856 | 753.3828 | 3.7 | 60.2 | |||||
| DHA | 720.3968 | 720.3950 | 2.5 | 68.1 | |||||
| VIHDNFGIVEGLMTTVHAITATQK | Oxidation | Unmodified | 871.1159 | 871.1215 | 6.4 | 57.7 | |||
| Unmodified | Oxidation | 876.4475‡ | 876.4535 | 6.8 | 51.2 |
Underlined letters represent site of modification.
CM, carbamidomethyl; Diox., dioxidation; m/z [Exp.], experimental mass-to-charge ratio; m/z [Theor.], theoretical mass-to-charge ratio; ppm, parts per million.
Tentative assignment based on Mascot search against human SwissProt database.
Highest Mascot score observed for the given modified peptide among all samples.
Both M175 sulfoxide and 2-oxo-H179 observed in the reported spectral match.
Two representative MS2 spectra of the peptide IISNASCTTNCLAPLAK of human GAPDH (residues 146-162). Shown are 2 irreversible modifications of the functional Cys152 (A) or His179 (B) in the active site pocket of the enzyme. Modifications were detected through error tolerant searches and Mascot searches including H,W oxidation and C oxidations as variable modifications. (A) Asterisk (*) on C156 indicates carbamidomethylation of unmodified or reversibly modified cysteine residues. In red and blue are highlighted y and b ions, respectively, that arise from the fragmentation of the modified peptides (35 eV CID collision energy).
Two representative MS2 spectra of the peptide IISNASCTTNCLAPLAK of human GAPDH (residues 146-162). Shown are 2 irreversible modifications of the functional Cys152 (A) or His179 (B) in the active site pocket of the enzyme. Modifications were detected through error tolerant searches and Mascot searches including H,W oxidation and C oxidations as variable modifications. (A) Asterisk (*) on C156 indicates carbamidomethylation of unmodified or reversibly modified cysteine residues. In red and blue are highlighted y and b ions, respectively, that arise from the fragmentation of the modified peptides (35 eV CID collision energy).
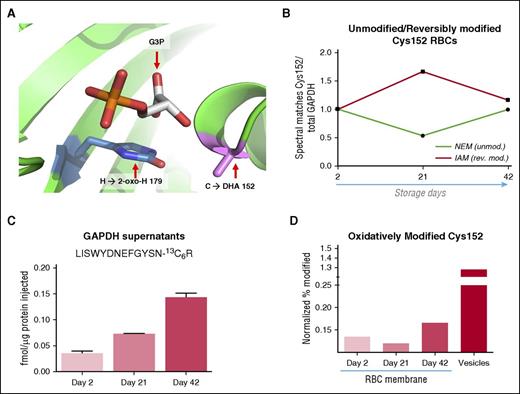
Oxidation of functional GAPDH residues and GAPDH vesiculation into supernatants. (A) A structural representation of GAPDH active site pocket, with Cys152 and His179 modified as to show 2 confidently assigned redox modifications (Cys to DHA; His to 2-oxo-His). (B) Ratios of NEM-tagged or IAM-tagged Cys152 (green and red continuous lines, respectively) vs total spectral matches for GAPDH in total RBC extracts at storage days 2, 21, and 42. Results represent a readout of unmodified or reversibly modified Cys152 GAPDH at lysis, respectively. (C) Absolute quantitation of GAPDH in stored RBC supernatants through QconCAT analysis. (D) Semiquantitative determination of oxidative modifications to Cys152 of GADPH are shown, calculated by determining the ratios of modified/total occurrences of confidently observed (P < .05) GAPDH peptide IISNASCTTNCLAPLAK, divided by the total number of all peptides confidently assigned for GAPDH.
Oxidation of functional GAPDH residues and GAPDH vesiculation into supernatants. (A) A structural representation of GAPDH active site pocket, with Cys152 and His179 modified as to show 2 confidently assigned redox modifications (Cys to DHA; His to 2-oxo-His). (B) Ratios of NEM-tagged or IAM-tagged Cys152 (green and red continuous lines, respectively) vs total spectral matches for GAPDH in total RBC extracts at storage days 2, 21, and 42. Results represent a readout of unmodified or reversibly modified Cys152 GAPDH at lysis, respectively. (C) Absolute quantitation of GAPDH in stored RBC supernatants through QconCAT analysis. (D) Semiquantitative determination of oxidative modifications to Cys152 of GADPH are shown, calculated by determining the ratios of modified/total occurrences of confidently observed (P < .05) GAPDH peptide IISNASCTTNCLAPLAK, divided by the total number of all peptides confidently assigned for GAPDH.
Switch-tag experiments were performed by preincubating stored RBCs with NEM to alkylate unmodified reduced cysteine thiols, prior to SDS-PAGE and tryptic digestion under reducing and alkylating conditions (eg, DTT and iodoacetamide [IAM]). This strategy affords determination of unmodified (supplemental Figure 1) or reversibly and irreversibly oxidized Cys residues.18,25 Results indicate a transient decrease of intracellular unmodified NEM-tagged Cys152 residues at storage day 21 (Figure 3B; supplemental Table 1). These residues indeed become reversibly oxidized (observed as IAM-modified; Figure 3B) at day 21, though the relative levels of reversibly oxidized Cys152 decrease by the end of the storage period, suggesting either decreased oxidation or vesiculation of oxidized GAPDH into supernatants.
By spiking in a heavy isotope-labeled peptide for GAPDH (LISWYDNEFGYSN(13C6)R), we determined the absolute abundance of GAPDH in RBC supernatants at storage days 2, 21, and 42 (Figure 3C) using a SRM-MS approach. Accumulation of GAPDH in the supernatant during storage illustrates the vesiculation process aimed at protecting the stored RBCs from damaged proteins. Progressive oxidation of Cys152 was observed in RBC membranes and day 42 vesicles (Figure 3D) as judged by the ratios of spectral matches for the irreversibly modified to reversible and unmodified tryptic peptides containing this residue, divided by the total number of peptide spectral matches to GAPDH. Taken together, these results suggest that irreversibly oxidized GAPDH content increases during RBC storage and is progressively vesiculated in RBC supernatants.
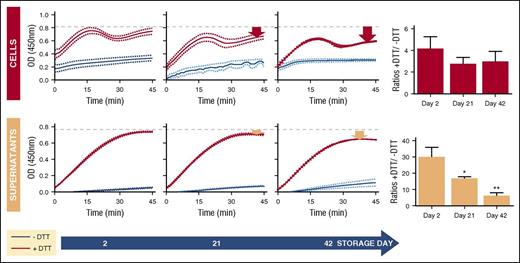
To confirm redox proteomics observations, enzymatic activity of cell and supernatant GAPDH were assayed in the presence or absence of the reducing agent DTT to assess the reversibility of the oxidation to functional GAPDH. Consistent with redox proteomics results, storage-dependent decreases in GAPDH activity were observed in both cells and supernatants (Figure 4). The relative percentage of activity that could be rescued by DTT decreased significantly at storage days 21 and 42, particularly in the supernatants (Figure 4).
Assessment of GAPDH enzymatic activity in total RBCs and RBC supernatants at storage days 2, 21, and 42. GAPDH activity was measured utilizing a commercial kit with detector molecule leading to increased absorbance at 450 nm in a manner directly proportional to GAPDH activity. The assay was carried out in the absence (blue lines) and presence (red lines) of DTT (2.5 mM). Bar plots at right represent the relative initial rates (0-10 minutes) of GAPDH catalysis ± DTT.
Assessment of GAPDH enzymatic activity in total RBCs and RBC supernatants at storage days 2, 21, and 42. GAPDH activity was measured utilizing a commercial kit with detector molecule leading to increased absorbance at 450 nm in a manner directly proportional to GAPDH activity. The assay was carried out in the absence (blue lines) and presence (red lines) of DTT (2.5 mM). Bar plots at right represent the relative initial rates (0-10 minutes) of GAPDH catalysis ± DTT.
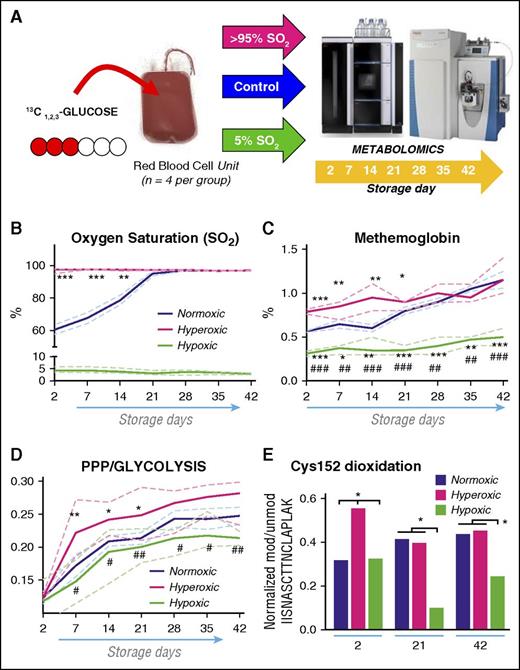
To test whether oxidative stress is the main driver of storage-dependent activation of the PPP and decreased GAPDH activity, metabolic flux analyses with 13C1,2,3-glucose were performed in leukocyte-filtered RBCs stored under control, high, or low oxygen saturation conditions (>95% and percentage of SO2, respectively) from day 1 (Figure 5). Analysis of oxidative stress parameters, such as MetHb content, revealed a higher initial MetHb percentage during the first 3 weeks of storage under hyperoxia, though MetHb increases were comparable in both groups after storage day 21 (Figure 5C). Hypoxia significantly decreased MetHb accumulation at all tested time points (Figure 5C). Consistently, flux analysis revealed that PPP was activated by all oxygen storage conditions, though significantly higher activation of the PPP was observed in hyperoxic RBCs throughout the whole storage period (Figure 5D). Although we previously documented reversible and irreversible oxidation of Hb in stored RBCs,26 here, redox proteomics analyses confirmed that irreversible oxidation of GAPDH Cys152 (thiol to sulfinic acid or β-elimination to DHA) was significantly lower in hypoxic RBCs and higher in hyperoxic RBCs at day 2 compared with normoxic conditions. However, hyperoxic and normoxic RBCs were comparable at day 21 and 42 (Figure 5E), when SO2 percentage, MetHb levels, and metabolic reprogramming to the PPP were not significantly different in the 2 groups. Improved preservation of GAPDH redox homeostasis in hypoxic RBCs correlated with significantly improved preservation of DPG and adenosine triphosphate (ATP) (supplemental Figure 2). Consistent with decreased oxidative stress in hypoxic RBCs, total glutathione pools were significantly higher in hypoxic RBCs in comparison with the other groups (supplemental Figure 3). Hypoxic RBCs were characterized by significantly higher than control de novo synthesis of glutathione, an ATP-dependent process, at least until storage day 28: as gleaned through flux analysis from 13C1,2,3-glucose and relative quantitation of glutathione precursors glutamine and cysteine (supplemental Figure 3).
Oxidative stress drives GAPDH irreversible modification and pentose phosphate pathway activation. (A) An overview of the metabolic flux analysis experiment, where four units were spiked with 11 mM 13C1,2,3-glucose and then stored either under normoxic (blue), hyperoxic (>95% SO2, magenta) or hypoxic (5% SO2, light green) conditions (n = 4 per group). (B) Hemoglobin oxygen saturation in normoxic red blood cells reached ∼95% by storage week 3, when 2,3-DPG reservoirs were totally consumed (supplemental Figure 2). (C) MetHb accumulation was higher in hyperoxic red blood cells during the first 3 storage weeks, before SO2 became comparable in both groups, whereas it remained significantly lower throughout storage duration in hypoxic red blood cells in comparison with normoxic and hyperoxic treatments. (D) PPP to glycolysis ratios were determined by dividing lactate isotopologues (+2/+3) at each tested time point (2-42, on a weekly basis). Continuous lines: median (blue, normoxic control; magenta, hyperoxic red blood cells); dashed lines: interquartile ranges. Although PPP remains active in all 3 groups, hyperoxic red blood cells are characterized by the highest activation rate. (E) Targeted SRM-based relative quantitation was performed for intracellular irreversibly oxidized Cys152 of GAPDH (normalized vs the relative abundance of the unmodified IISNASCTTNCLAPLAK peptide containing the active site residue) in normoxic, hyperoxic, and hypoxic red blood cells at storage day 2, 21, and 42. Significantly higher levels of irreversibly oxidized Cys152 of GAPDH were determined at storage day 2 but not at later time points, when SO2, methemoglobin levels and PPP:glycolysis ratios were comparable in either groups. * and # indicate significance (P < .05; < 0.01; < 0.001) vs normoxic controls or hyperoxic units, respectively.
Oxidative stress drives GAPDH irreversible modification and pentose phosphate pathway activation. (A) An overview of the metabolic flux analysis experiment, where four units were spiked with 11 mM 13C1,2,3-glucose and then stored either under normoxic (blue), hyperoxic (>95% SO2, magenta) or hypoxic (5% SO2, light green) conditions (n = 4 per group). (B) Hemoglobin oxygen saturation in normoxic red blood cells reached ∼95% by storage week 3, when 2,3-DPG reservoirs were totally consumed (supplemental Figure 2). (C) MetHb accumulation was higher in hyperoxic red blood cells during the first 3 storage weeks, before SO2 became comparable in both groups, whereas it remained significantly lower throughout storage duration in hypoxic red blood cells in comparison with normoxic and hyperoxic treatments. (D) PPP to glycolysis ratios were determined by dividing lactate isotopologues (+2/+3) at each tested time point (2-42, on a weekly basis). Continuous lines: median (blue, normoxic control; magenta, hyperoxic red blood cells); dashed lines: interquartile ranges. Although PPP remains active in all 3 groups, hyperoxic red blood cells are characterized by the highest activation rate. (E) Targeted SRM-based relative quantitation was performed for intracellular irreversibly oxidized Cys152 of GAPDH (normalized vs the relative abundance of the unmodified IISNASCTTNCLAPLAK peptide containing the active site residue) in normoxic, hyperoxic, and hypoxic red blood cells at storage day 2, 21, and 42. Significantly higher levels of irreversibly oxidized Cys152 of GAPDH were determined at storage day 2 but not at later time points, when SO2, methemoglobin levels and PPP:glycolysis ratios were comparable in either groups. * and # indicate significance (P < .05; < 0.01; < 0.001) vs normoxic controls or hyperoxic units, respectively.
Discussion
Storage of packed erythrocytes for transfusion therapy is a logistic necessity to supply millions of blood units for life-saving interventions every year. Despite a century of improvements in the field27 and reassuring prospective clinical evidence about the safety and efficacy of longer stored units,28-30 the field of transfusion medicine is still proactively committed to improving cell-processing and storage strategies.31,32 Improving our understanding of the storage lesion is a key first step to fulfill this ambitious agenda.32 Loss of metabolic modulation of stored erythrocytes, that is, an impaired capacity to preserve energy and redox poise, is a key central component of the storage lesion.5,6
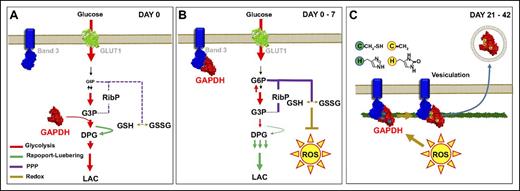
Here, energy metabolism was monitored through UHPLC-MS during routine storage of leukocyte-filtered erythrocyte concentrates in AS-3 additive solution, one of the most common additives in the United States.11,33 Metabolomics results were consistent with previous reports,5,9 showing a progressive deregulation of energy metabolism after 2 weeks of storage. Consumption of high-energy phosphate compounds such as 2,3-DPG without net loss of upstream metabolite glyceraldehyde 3-phosphate suggests a decrease in GAPDH activity in stored RBCs.15 GAPDH activity, which provides 1,3-DPG as a substrate for the Rapoport-Luebering shunt reaction that generates 2,3-DPG via biphosphoglycerate mutase, is dependent on its relocation to the membrane. At the membrane level, binding of the negatively charged N-terminal cytosolic domain of band 323 to the active site pocket of GAPDH compromises GAPDH glycolytic activity (Figure 6B).7 However, though membrane binding of GAPDH in stored RBCs transiently increases between the second and third storage week, by storage day 21 GAPDH membrane levels decrease again, as previously reported in RBCs stored in SAGM-additive solution.15 Oxidative modifications to functional residues of cytosolic GAPDH have thus been suggested to adversely affect its activity, though only the reversible modulation of activity via an intramolecular disulfide bond between Cys152 and 156 has been reported.15
A representative model of GAPDH-dependent metabolic modulation in stored RBCs. For simplicity, GAPDH monomers are shown. The oxidative lesion may affect oxygen-dependent metabolic modulation, promoting GAPDH binding to the N-term or other residues of band 3, promoting a metabolic shift from Embden-Meyerhof to the PPP as to generate reducing equivalents (NADPH) and attempt to restore the redox poise. As RBCs approach the end of the storage period, oxidative stress becomes overwhelming and irreversible oxidative lesions accumulate in GAPDH, and as previously reported, Hb.26 Irreversibly oxidized proteins are thus selectively vesiculated in RBC supernatants, a potential protective mechanism.40 ROS, reactive oxygen species.
A representative model of GAPDH-dependent metabolic modulation in stored RBCs. For simplicity, GAPDH monomers are shown. The oxidative lesion may affect oxygen-dependent metabolic modulation, promoting GAPDH binding to the N-term or other residues of band 3, promoting a metabolic shift from Embden-Meyerhof to the PPP as to generate reducing equivalents (NADPH) and attempt to restore the redox poise. As RBCs approach the end of the storage period, oxidative stress becomes overwhelming and irreversible oxidative lesions accumulate in GAPDH, and as previously reported, Hb.26 Irreversibly oxidized proteins are thus selectively vesiculated in RBC supernatants, a potential protective mechanism.40 ROS, reactive oxygen species.
Recently, it has been argued that not all membrane-bound GAPDH is inactive because binding to other domains of band 3 (either C-terminus or residues 156-184) or structural proteins, such as ankyrin, α/β-spectrin, protein 3.2, or actin, does not affect GAPDH activity.7 In light of these observations, we asked whether oxidative modifications at key GAPDH residues could be observed in the membrane-bound subpopulation of this enzyme. Redox modifications are substoichiometric and often reversible, challenging their detection in vivo through proteomics technologies without exogenous supplementation of excess concentrations (eg, ≥1 mM) of pro-oxidants like diamide or H2O2.2,4 However, reactive oxygen species accumulation and oxidation of proteins in stored RBCs, as gleaned through dinitrophenylhydrazine reactivity assays, lipid assays (eg, malondialdehyde), and proteomics analysis of protein aggregation/fragmentation events are reportedly higher than physiological values.34-36 Here we report for the first time ex vivo irreversible oxidations of redox-sensitive residues Cys152, 156, and 247, and His165 and 179 in the membrane-bound or day 42 vesicle fractions. Through a combination of switch-tag redox18,25 and quantitative proteomics,17,37 we identify the transient accumulation of reversible oxidation in stored RBC membranes and the progressive accumulation of irreversibly oxidized GAPDH in stored RBC supernatants. In particular, irreversible cysteine to DHA conversion was detected for all 3 functional cysteines 152, 156, and 247, an observation that was accompanied by the progressive loss of DTT-dependent reversible activity of GAPDH, especially in supernatants (Figure 6C).
GAPDH activity is mediated by the nucleophilic attack of the Cys152 thiolate on the carbonyl of glyceraldehyde 3-phosphate, resulting in hemiacetal formation. Dioxidation of Cys152 and 156 would introduce negative charges in the active site of GAPDH, thereby potentially disrupting electrostatic interactions between the catalytic pocket and the negatively charged N terminus of band 3.23 Similarly, Cys to DHA conversion destroys the nucleophilicity of Cys152, rendering the enzyme irreversibly inactive and perhaps interfering with band 3 binding, according to the GAPDH/band 3-interaction model proposed by Eisenmesser and Post.23
Though distant from the active site, Cys247 has key regulatory functions in GAPDH as it represents a target for S-nitrosation in RBCs.3 Of note, impaired S-nitrosothiol signaling is reportedly associated with a reduced vasodilatory capacity of old, stored RBCs.5 GAPDH Cys247 is also a target of oxidation in peroxiredoxin 2 knockout mice.3 Similarly, long-stored RBCs are characterized by progressive oxidation and membrane translocation of oxidized peroxiredoxin 2, a biochemical lesion that underlies an increasing impairment of redox homeostasis during ex vivo preservation of packed erythrocytes.5,26,38,39
Although RBC vesiculation had been proposed as a self-protective mechanism in erythrocytes aging ex vivo to eliminate irreversibly damaged components,40 evidence only related to increased carbonylation in vesiculated proteins had been produced so far.34,35 Vesiculation in response to other irreversible oxidative modifications, such as on low redox potential residues like Cys, has not been described. Here we show that oxidized and significantly less active GAPDH progressively accumulates in stored RBC supernatants. In the absence of significant hemolysis (<0.2% for all tested units), a likely mechanism explaining this observation is vesiculation,40-42 as confirmed by ultracentrifugation-mediated enrichment of RBC-shed vesicles at storage day 42. Extracellular or vesiculated GAPDH has been implicated in the scavenging of transferrin-bound iron.3 Such observations may underlie an unappreciated RBC adaptation to cope with potential free radical damage ensuing upon hemolytic release of heme iron during in vivo, or in this case ex vivo, aging.32 The proteomics workflows used here ultimately included reduction and alkylation steps prior to digestion and thus do not permit the direct observation of disulfide bonds. Generation of intermolecular disulfides underlies reversible protein multimerization, a phenomenon observed in oxidative stress–associated pathologies such as Alzheimer disease.43 However, no significant multimerization of GAPDH has been observed in native proteomics studies of RBC multiprotein complexes during routine storage in SAGM.44
Application of a quantitative metabolic flux analysis workflow allowed us to determine a significant activation of the PPP in leukocyte-filtered AS-3 RBCs after storage day 21. This mechanism is consistent with previous observations in cellular models, where exogenous oxidative stress promoting GAPDH reversible oxidation results in the activation of the hexose monophosphate shunt pathway in order to generate reducing equivalents (NADPH) and restore the redox poise.1,45-47 The contrast with early nuclear magnetic resonance spectroscopy metabolomics studies8 could be explained by the appreciation of the beneficial effects of RBC storage upon leukocyte removal by filtration12,13,48 and the implementation of novel and more effective additives than SAGM,10,11,33 both factors limiting the proteomics and metabolomics storage lesion. To complement observational correlative evidence, owing to the biological limitations of the model (not amenable to mechanistic genetic intervention or GAPDH activity inhibition with small molecule compounds such as koningic acid for up to 7 weeks49 ), we showed that storage of RBCs under hyperoxic conditions (>95% SO2) triggered a significantly higher accumulation of MetHb and activation of the PPP relative to normoxic conditions only during the first 3 weeks of storage. After storage day 14, when 2,3-DPG reservoirs in control units were exhausted and the percentage of SO2 increased to levels comparable to hyperoxic RBCs, the normoxic and hyperoxic groups were comparable in terms of MetHb content, PPP activation, and irreversible intracellular GAPDH oxidation. These results suggest that full oxygen saturation of Hb and irreversible oxidation of Hb Cys and His residues, as we recently reported,26 likely drive oxidation of proteins involved in or regulated by cellular redox dynamics, such as GAPDH or peroxiredoxin 2, respectively. Of note, recycling of oxidized peroxiredoxin 2 cysteines is inhibited during erythrocyte storage, a phenomenon that is linked to the irreversible oxidation of Cys93 of Hb β to DHA,50 a recently appreciated modification to stored RBC proteins.26,50
On the other hand, hypoxic storage of RBCs prevented GAPDH oxidation and preserved RBC energy metabolic homeostasis without fully eliminating metabolic fluxes through the PPP, despite previous inferences from steady-state metabolomics,51 ultimately resulting in improved de novo synthesis of glutathione (an ATP-dependent process) until storage day 28 and preserved glutathione homeostasis through the storage period. These results expand and support previous evidence of the beneficial role of hypoxic storage on the energy, redox, and morphological lesions to packed RBCs.24,52,53
Metabolic flux analyses also provided supporting evidence to reconcile contrasting observations about the substantial preservation of the activity of glucose 6-phosphate dehydrogenase (G6PD), the rate-limiting enzyme of the oxidative branch of the PPP, in leukocyte-filtered RBCs stored in AS-3.54,55 Previous observations about the apparent decrease in the enzymatic activity of G6PD55 may be explained by (1) the decreased activity of G6PD at blood bank storage temperature (2°C-6°C); (2) a response to declines in intracellular pH, as a side effect of ongoing glycolysis; (3) a response specific to SAGM RBCs.55,56
Conclusion
Previous reports of metabolic regulatory redox modifications1 in RBCs were achieved only in the presence of excess concentrations of pro-oxidants such as diamide and H2O2.2,4 Quantitative proteomics and metabolomics approaches were here combined to uncover multiple oxidative lesions in the active site of GAPDH in stored erythrocytes without supplementation of exogenous pro-oxidant compounds. GAPDH oxidation correlated with impaired glycolysis and increased fluxes through the PPP to generate reducing equivalents and restore the redox poise. In the absence of significant hemolysis (<0.4% in all tested units), vesiculation into stored RBC supernatants of a subpopulation enriched in irreversibly oxidized GAPDH is documented. A novel quantitative metabolic flux analysis workflow is described using a combination of isotope tracing of 13C1,2,3-glucose57 and heavy labeled standards.20,58 The workflow is used to absolutely quantify the shift from glycolysis to the PPP by determining lactate isotopologue ratios. Although the mechanism proposed here was inferred through correlative evidence, RBC storage under hyperoxic and hypoxic conditions mechanistically exacerbated or prevented the energy and oxidative RBC storage lesion, respectively, suggesting that alternative strategies may be already available to ameliorate storage quality.
Further studies will assess whether oxidized GAPDH in stored RBC supernatants switches from canonical metabolic functions to alternative moonlighting functions, such as scavenging of transferrin-bound iron.3,59,60 Owing to the evolutionary conservation and ubiquitous expression of GAPDH, future studies using the technology described here will assess whether the proposed model may be applicable to other biologically relevant matrices, such as oxidative stress–challenged RBCs (eg, sickle cells61 ), RBCs in aging, septic shock or Down syndrome, or highly proliferating cancer cells.
This article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
A.D. received research support from the National Blood Foundation (Early Career Grant, grant cycle 2016).
Authorship
Contribution: J.A.R. and A.D. designed the experiments; J.A.R., M.J.W., M.D., R.C.H., and K.C.H. performed either quantitative or redox proteomics experiments, analyzed proteomics results, or critically contributed to the realization of the proteomics experiments; T.Y. and A.D. designed metabolomics experiments; T.Y., A.J.D., and A.D. designed the hyperoxic and hypoxic storage experiments; T.N. and A.D. performed metabolomics experiments; A.I. prepared GAPDH structure models and related figures; A.D., M.J.W., and J.A.R. prepared the figures, while J.A.R. and M.J.W. prepared the tables and supplemental material; A.D. and J.A.R. wrote the paper; and all of the authors critically contributed to the finalization of the manuscript.
Conflict-of-interest disclosure: T.Y. and A.J.D. are employed by New Health Sciences, Inc. A.D., K.C.H., R.C.H. and T.N. are founders of Endura, LLC. A.D. is a consultant for NHSi. The remaining authors declare no competing financial interests.
Correspondence: Angelo D’Alessandro, Department of Biochemistry and Molecular Genetics, University of Colorado Denver, Anschutz Medical Campus, Aurora, CO 80045; e-mail: angelo.dalessandro@ucdenver.edu.