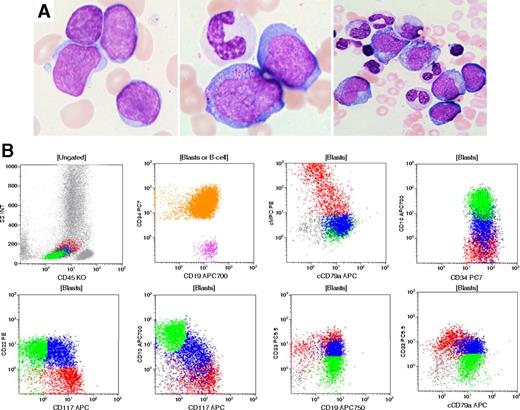
A 77-year-old man presented with a past history of gout, hypothyroidism, and persistent thrombocytopenia. Bone marrow examination revealed megakaryocytic hypoplasia and no significant dysplasia. The karyotype was normal. Six years later, he developed severe pancytopenia with rare blasts in peripheral blood. A bone marrow aspirate revealed 34% blasts. Morphologically, blasts varied in size and some showed cytoplasmic granules and abundant cytoplasm (panel A). Erythropoiesis and megakaryopoiesis were markedly reduced, and granulocytic lineage showed prominent dysplasia. Flow cytometry demonstrated a substantial population of CD34+ blasts (panel B, orange shading) that exhibited a complex pattern of lineage-associated markers. One subset expressed B-lineage markers (green): CD19+, CD10+, CD22+, cCD79a+, TdT+, also positive for CD33(dim), CD13, negative for MPO, cCD3; and the other expressed a predominantly myeloid phenotype (red): MPO+, CD117+, CD33+ (bright), CD13+, CD19+, TdT+, cCD79a dim/negative, CD10–, CD22–, cCD3–. An intermediate population could also be detected (blue). Cytogenetic studies showed a complex karyotype: 48XY, –7, +21, +21, +22. Molecular studies for BCR-ABL1 fusion gene were negative.
The diagnosis of acute myeloid leukemia with myelodysplasia-related changes was made and the mixed phenotype of blasts was noted. The final diagnosis in this rare case reflected the current World Health Organization classification in the settings of a complex karyotype including monosomy 7.
A 77-year-old man presented with a past history of gout, hypothyroidism, and persistent thrombocytopenia. Bone marrow examination revealed megakaryocytic hypoplasia and no significant dysplasia. The karyotype was normal. Six years later, he developed severe pancytopenia with rare blasts in peripheral blood. A bone marrow aspirate revealed 34% blasts. Morphologically, blasts varied in size and some showed cytoplasmic granules and abundant cytoplasm (panel A). Erythropoiesis and megakaryopoiesis were markedly reduced, and granulocytic lineage showed prominent dysplasia. Flow cytometry demonstrated a substantial population of CD34+ blasts (panel B, orange shading) that exhibited a complex pattern of lineage-associated markers. One subset expressed B-lineage markers (green): CD19+, CD10+, CD22+, cCD79a+, TdT+, also positive for CD33(dim), CD13, negative for MPO, cCD3; and the other expressed a predominantly myeloid phenotype (red): MPO+, CD117+, CD33+ (bright), CD13+, CD19+, TdT+, cCD79a dim/negative, CD10–, CD22–, cCD3–. An intermediate population could also be detected (blue). Cytogenetic studies showed a complex karyotype: 48XY, –7, +21, +21, +22. Molecular studies for BCR-ABL1 fusion gene were negative.
The diagnosis of acute myeloid leukemia with myelodysplasia-related changes was made and the mixed phenotype of blasts was noted. The final diagnosis in this rare case reflected the current World Health Organization classification in the settings of a complex karyotype including monosomy 7.
For additional images, visit the ASH IMAGE BANK, a reference and teaching tool that is continually updated with new atlas and case study images. For more information visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal