Key Points
Both Notch1 and Notch2 receptors are involved in pre-HSC maturation.
Developing HSCs become Notch independent by the end of their maturation in the AGM region.
Abstract
The first definitive hematopoietic stem cells (dHSCs) in the mouse emerge in the dorsal aorta of the embryonic day (E) 10.5 to 11 aorta-gonad-mesonephros (AGM) region. Notch signaling is essential for early HSC development but is dispensable for the maintenance of adult bone marrow HSCs. How Notch signaling regulates HSC formation in the embryo is poorly understood. We demonstrate here that Notch signaling is active in E10.5 HSC precursors and involves both Notch1 and Notch2 receptors, but is gradually downregulated while they progress toward dHSCs at E11.5. This downregulation is accompanied by gradual functional loss of Notch dependency. Thus, as early as at final steps in the AGM region, HSCs begin acquiring the Notch independency characteristic of adult bone marrow HSCs as part of the maturation program. Our data indicate that fine stage-dependent tuning of Notch signaling may be required for the generation of definitive HSCs from pluripotent cells.
Introduction
In the mouse embryo, the first definitive hematopoietic stem cells (dHSCs), capable of long-term multilineage engraftment in the irradiated adult recipient, emerge in the floor of the dorsal aorta within the aorta-gonad-mesonephros (AGM) region around late embryonic day (E) 10.5 to 11.1-4 HSC development is closely linked to the appearance of intra-aortic hematopoietic cell clusters observed in various vertebrate species, including humans.5-13 Coexpression of endothelial and hematopoietic markers and transcription factors in cluster cells suggests emergence of HSCs and progenitor cells from the underlying hematogenic endothelium13-17 through a Runx1-dependent process.18-23 Recent observations indicate that the emergence of HSCs involves expansion and gradual maturation of embryonic precursors, termed pre-HSCs, which express an endothelial marker vascular endothelial–cadherin (VC) and sequentially upregulate hematopoietic markers such as CD41, CD43, and CD45. Pro-HSCs (VC+CD45−CD41+CD43−) detected in E9.5 embryos mature into pre-HSC type I (VC+CD45−CD41+CD43+) in E10.5 AGM and then into pre-HSC type II (VC+CD45+CD41+CD43+) which are mainly present at E11.5.24-29 In contrast to dHSCs, these precursors are not detectable by direct transplantations into adult irradiated recipients. A maturation step in an embryonic or neonatal environment is needed to allow them to develop into transplantable dHSCs.24-27
The Notch pathway is involved in numerous biological processes such as cell-fate decisions, stem cell homeostasis, proliferation, and apoptosis.30,31 Interactions of Notch receptors with ligands (in mammals, Notch1-4 and Jag1-2, Dll1, 4, respectively) release the Notch intracellular domain, which, through collaboration with the RBP-Jκ transcription factor, activates Notch targets such as transcriptional repressor Hes1.32 Notch plays an important role in embryonic HSC development33-35 but is dispensable for adult bone marrow HSCs.36,37 Notch1 mutant embryonic stem (ES) cells fail to contribute to adult hematopoiesis, suggesting its cell-autonomous role in HSC specification.38 Notch signaling is required for specification of the hematogenic endothelium in the lateral plate mesoderm39-41 and for establishing arterial identity of the endothelium, closely related to the hematopoietic specification.33,42-46 Mouse Notch1, Jag1, or RBP-Jκ mutants are embryonic lethal and exhibit severely impaired hematopoiesis concurrent with expansion of the aortic endothelial cell population, suggesting regulation of the hematogenic endothelium fate by Notch1-Jag1 signaling.33-35 Notch2 knockouts show no obvious hematopoietic defects33 and Notch3 and Notch4 knockouts are viable, indicating their nonessential role in HSC development.43,47 The requirement for Notch in the endothelial-hematopoietic transition is conserved in zebrafish,19,48-51 where Notch1 acts through activation of and cooperation with important transcription factors such as Gata2, Runx1, Scl, Foxc2, and Hes1/5.34,48,50-54 Although Notch is essential for early HSC development, exact stage-specific requirements for this signaling pathway in this multistep maturation process remain unclear.
Here, we show that although Notch signaling is active in and critical for pre-HSC development, downregulation of Notch activity during transition from the pre-HSC type I to the type II stage is essential for this process. However, Notch signaling is largely dispensable for the next step of maturation of pre-HSC type II into dHSCs in the AGM region. Although Notch1 is the dominant Notch receptor player, Notch2 also contributes to pre-HSC development. Thus, consistently with the acquisition of the adult status, developing HSCs in the AGM region gain Notch independency, which is a hallmark of adult bone marrow HSCs.36
Materials and methods
Mice
Wild-type and transgenic mouse lines (all C57BL/6, CD45.2/2) used were: (1) a pHes1-d2EGFP reporter of Hes1 expression,55 (2) RosaCreERT2 (from L. Grotewold and A. Smith, Wellcome Trust Centre for Stem Cell Research, University of Cambridge, Cambridge, UK), (3) sGFP where green fluorescent protein (GFP) is expressed upon Cre-mediated activation,56 and (4) floxed RBP-Jκ.37 The following primers were used for genotyping by polymerase chain reaction (PCR): (1) RBP-Jκ as in Souilhol et al57 ; (2) RosaCreERT2: F1-AAAGTCGCTCTGAGTTGTTAT, R1-GGAGCGGGAGAAATGGATATG, R2-CATCAAGGAAACCCTGGACTACTG (wild-type allele = 582 bp, RosaCreERT2 allele = 249 bp); (3) sGFP: F1-AAGTTCATCTGCACCACCG, R1-TCCTTGAAGAAGATGGTGCG (173 bp); (4) Hes1-GFP: F1-TCACACAGGATCTGGAGCTG, R1-GAACTTCAGGGTCAGCTTGC (250 bp). Mice were housed and bred in animal facilities at the University of Edinburgh, in compliance with the Home Office regulations. All experiments with animals were conducted under a Home Office UK Project License and approved by the University of Edinburgh Ethical Review Committee.
Cultures
Reaggregates and explants were cultured at the liquid-gas interface. Pre-HSC populations sorted from 1 AGM region were coaggregated with 105 OP9 cells and cultured on filter for 5 days in the presence of cytokines (interleukin-3 plus stem cell factor plus Flt3 ligand; PeproTech) as previously described.25,26 After culture, single-cell suspensions prepared by dispase/collagenase digestion were used for colony-forming unit culture (CFU-C) and transplantation assays and fluorescence-activated cell sorter (FACS) analysis.26
Cre-mediated recombination of the RBP-Jflox allele was induced by addition of 4-hydroxytamoxifen (4-OHT) (5 μM; Sigma) to cell suspensions for 2 hours at 37°C before culture.
For blocking Notch, freshly isolated AGM cell suspensions were incubated before culture with N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) (Calbiochem), or Notch1- and Notch2-blocking antibodies (anti-NRR1 and anti-NRR2; Genentech) for at least 20 minutes at 37°C. DAPT (50 μM) or blocking antibodies (10 μg/mL) were also added to the medium at the beginning of the culture.
Transplantations
Embryonic single-cell suspensions (CD45.2/2) were injected into adult recipients (CD45.1/2) along with 20 000 CD45.1/1 bone marrow carrier cells. Recipients were irradiated by split dose (600 plus 550 rad with 3-hour interval) of γ-irradiation. The cell numbers of a particular population are expressed in doses, defined as embryo equivalent (ee), which corresponds to the number of given cells in 1 AGM region (for example, 0.2 ee is equal to 20% of a given cell population in 1 AGM region). Donor-derived chimerism was monitored in blood at 6 and 14 weeks after transplantation by FACS. Peripheral blood cells treated with PharmLyse were stained with anti-CD16/32, anti-CD45.1-allophycocyanin (clone A20), and anti-CD45.2-phycoerythrin (clone 104) antibodies (eBioscience). Different groups of repopulated mice were compared using Mann-Whitney statistical tests.
Flow cytometry
Flow cytometry was performed using FACSCalibur and Fortessa (analysis) and FACSAria II or FACSAria Fusion, using FACSDiva software (sorting). Data were analyzed in FlowJo software (TreeStar). The antibodies used are listed in supplemental Table 1 (available on the Blood Web site). Cell viability was assessed using 7-amino-actinomycin D (7-AAD) or ethidium monoazide (EMA). Gates were set using fluorescence minus one (FMO) controls. All stainings except for RBP-Jκ were performed on live cells. For intracellular RBP-Jκ staining, cells were fixed in 2% paraformaldehyde for 10 minutes at 4°C and incubated for 30 minutes in 50% fetal calf serum, 0.4% Tween 20 before antibody staining.
Hematopoietic colony assay
Fresh or cultured live cells were seeded into methylcellulose supplemented with cytokines (M3434; StemCell Technologies) for 8 to 12 days to assess the presence of myeloid colony-forming cells (CFU-Cs). To determine the genotype of RBP-JCKO dHSCs, 20 000 to 40 000 cells isolated from recipient bone marrow were seeded into methylcellulose after culture colonies were individually picked and genotyped to detect RBP-Jflox and RBP-JΔ alleles.
Dll1 doxycycline-inducible OP9 cell line (OP9-Dll1)
Delta-like1 (Dll1) complementary DNA (cDNA) was cloned into a doxycycline-inducible bicistronic expression vector pPBhCMV1-cHA-IRESVenuspA (gift from H. Niwa, Institute of Molecular Embryology and Genetics, Department of Pluripotent Stem Cells, University of Kumamoto, Kumamoto, Japan58 ) that allowed both Dll1 and Venus to be expressed upon induction. One hundred thousand OP9 cells were electroporated with this construct using the NEON transfecting system (Invitrogen). The next day, the electroporated cells were cultured in the presence of 1 µg × mL−1 doxycycline (Clontech). The cells which showed no background Venus expression were sorted and used for coaggregation experiments. Before coaggregation with AGM cells, OP9-Dll1 cells were precultured with 1 µg × mL−1 doxycycline for 24 hours. Coaggregates were cultured in the presence of doxycycline for 5 days.
qRT-PCR
Total RNA was isolated from AGM and OP9 with the RNeasy microkit or minikit (Qiagen), respectively, and cDNA prepared using SuperScriptVILO cDNA Synthesis (Invitrogen). The quantitative reverse transcription PCR (qRT-PCR) was performed using the Light Cycler 480 SYBR Green I Master Mix (Roche). For a small number of cells, total RNA was isolated from 200 cells directly FACS sorted and cDNA prepared using CellsDirect (Invitrogen). qRT-PCR was performed using a universal probe library (UPL) and the Lightcycler 480 probes mastermix kit (Roche) in duplicates. Expression values were normalized against the TATA-binding protein (TBP) and standard errors were calculated and plotted using Prism6 software (GraphPad). Primer sequences are available on supplemental Table 2.
Results
Notch signals in pre-HSCs and dHSCs
To analyze Notch signaling in the developing HSC lineage, we first examined expression of Notch receptors. qRT-PCR showed that both Notch1 and Notch2 are expressed in phenotypically defined pre-HSC type I (VC+CD45−CD43+) and type II (VC+CD45+) populations in the E11.5 AGM region, whereas Notch3 and Notch4 are expressed at lower, sometimes negligible, levels (Figure 1A). Accordingly, immunophenotyping by flow cytometry showed that Notch1 is highly expressed in most endothelial (VC+CD45−CD41−) cells (>90%) and in all pre-HSC type I (VC+CD45−CD41+) (Figure 1B). Notch1 expression then decreases in the pre-HSC type II (VC+CD45+) population so that 30% of them become Notch1− (Figure 1B). Conversely, although a minority of endothelial cells and pre-HSC type I express Notch2 (16.2% and 22%, respectively), >70% of pre-HSC type II become Notch2+ (Figure 1B; supplemental Table 3). We then investigated whether functional pre-HSCs express Notch1 and Notch2 by using the OP9 coculture system, which allows pre-HSCs to mature into detectable HSCs.24,25 Functional analysis using sorted Notch1+ and Notch1− cells from the E11.5 AGM region followed by coaggregation with OP9 cells and transplantation into irradiated recipients confirmed that Notch1 is expressed in all pre-HSCs (both type I and type II) because only Notch1+ cells were able to generate dHSCs (Figure 1C). Similar functional tests showed that some pre-HSC type II also express Notch2 (Figure 1D; supplemental Figure 1A-B). Direct transplantations showed that dHSCs from the E11.5 AGM region and E12.5 fetal liver can be found in both Notch1low/high and Notch2low/high fractions (supplemental Figure 1C-D).
Expression of Notch receptors in HSC lineage in AGM region. (A) Expression levels of Notch receptors assessed by qRT-PCR in endothelial cells (VC+CD45−CD43−), pre-HSC type I (VC+CD45−CD43+) and pre-HSC type II (VC+CD45+) sorted from the E11.5 AGM region (n = 3). Data are mean ± standard error of the mean (s.e.m.). *P < .05, **P < .01. (B) Expression of Notch1 and Notch2 in HSC lineage. FACS analysis representing Notch1 or Notch2 presence at the surface of endothelial cells (VC+CD45−CD41−), pre-HSC type I (VC+CD45−CD41+) and pre-HSCs type II (VC+CD45+) in the E11.5 AGM region (n = 3). The graphs on the right indicate mean fluorescence intensity (MFI) ratios between Notch1 or Notch2 and their respective FMO controls during the endothelial-to-pre-HSC transition in 3 independent experiments. Data are mean ± s.e.m. *P < .05. (C) All functional pre-HSCs express Notch1. E11.5 AGM cells were sorted based on Notch1 expression and 2 populations (Notch1+ and Notch1−) were coaggregated with OP9 cells and cultured for 5 days before transplantation into irradiated mice in order to functionally assess the presence of pre-HSCs (0.5 ee per recipient). n = 2; *P < .05, Mann-Whitney U test. (D) Notch2 is expressed in functional pre-HSC type II, but not in pre-HSC type I. E11.5 VC+CD45− cells (type I) and VC+CD45+ (type II) were sorted based on Notch2 expression level and coaggregated with OP9. After 5 days of culture, they were injected into irradiated recipients (pre-HSC type I: 1 ee per recipient; pre-HSC type II: 0.1 ee per recipient); n = 2; ***P < .005, Mann-Whitney U test. ns, nonsignificant, t test.
Expression of Notch receptors in HSC lineage in AGM region. (A) Expression levels of Notch receptors assessed by qRT-PCR in endothelial cells (VC+CD45−CD43−), pre-HSC type I (VC+CD45−CD43+) and pre-HSC type II (VC+CD45+) sorted from the E11.5 AGM region (n = 3). Data are mean ± standard error of the mean (s.e.m.). *P < .05, **P < .01. (B) Expression of Notch1 and Notch2 in HSC lineage. FACS analysis representing Notch1 or Notch2 presence at the surface of endothelial cells (VC+CD45−CD41−), pre-HSC type I (VC+CD45−CD41+) and pre-HSCs type II (VC+CD45+) in the E11.5 AGM region (n = 3). The graphs on the right indicate mean fluorescence intensity (MFI) ratios between Notch1 or Notch2 and their respective FMO controls during the endothelial-to-pre-HSC transition in 3 independent experiments. Data are mean ± s.e.m. *P < .05. (C) All functional pre-HSCs express Notch1. E11.5 AGM cells were sorted based on Notch1 expression and 2 populations (Notch1+ and Notch1−) were coaggregated with OP9 cells and cultured for 5 days before transplantation into irradiated mice in order to functionally assess the presence of pre-HSCs (0.5 ee per recipient). n = 2; *P < .05, Mann-Whitney U test. (D) Notch2 is expressed in functional pre-HSC type II, but not in pre-HSC type I. E11.5 VC+CD45− cells (type I) and VC+CD45+ (type II) were sorted based on Notch2 expression level and coaggregated with OP9. After 5 days of culture, they were injected into irradiated recipients (pre-HSC type I: 1 ee per recipient; pre-HSC type II: 0.1 ee per recipient); n = 2; ***P < .005, Mann-Whitney U test. ns, nonsignificant, t test.
To analyze Notch activity in the developing HSC lineage, we used reporter mice in which a destabilized GFP is driven by the Hes1 promoter.55 We found that Hes1-GFP is expressed in subsets of the endothelial population and of phenotypically defined pre-HSC type I and type II populations in the E11.5 AGM region (Figure 2A). qRT-PCR analysis showed quantitative correlation between Hes1 and GFP transcript levels both in pre-HSC type I and type II populations (supplemental Figure 2A). Hes1-GFP+ pre-HSCs showed an enrichment in expression of other Notch target genes, such as Hey1 and Hey2 (supplemental Figure 2A). As expected, addition of the Notch/γ-secretase inhibitor, DAPT, efficiently downregulated Hes1-GFP in both endothelial and pre-HSC populations within 24 hours (supplemental Figure 2B; J.G.L., unpublished data). Conversely, addition of doxycycline to coaggregates with inducible OP9-Dll1 cells (supplemental Figure 3A) elevated Hes1-GFP expression significantly in pre-HSC type I and to a lesser extent in pre-HSC type II (Figure 3A-B). Although Jag1, a weak inducer of Notch activity,59 is expressed in OP9-WT cells (supplemental Figure 3B), this was not sufficient to maintain Hes1-GFP expression in pre-HSCs (Figure 3A-B), indicating that this GFP reporter may not reveal weak Notch signaling.
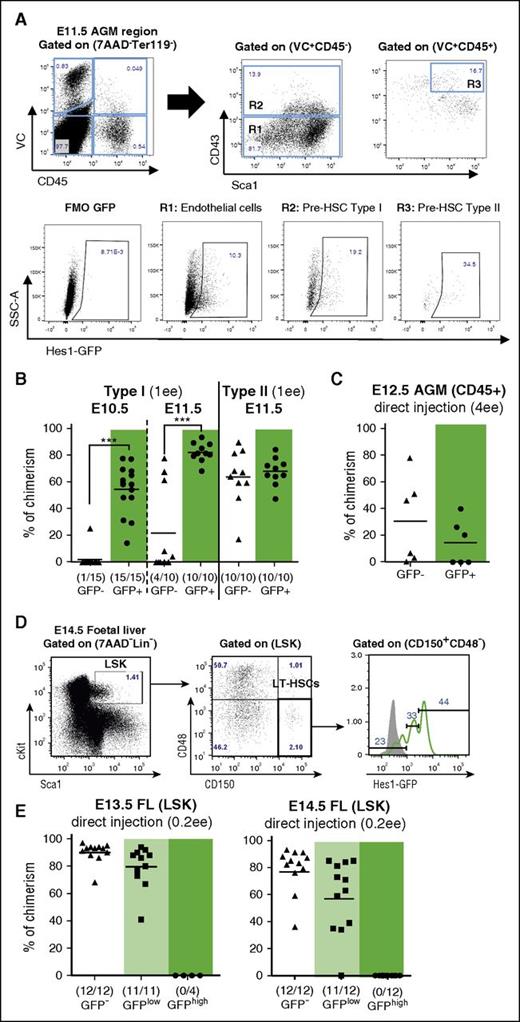
Notch activity decreases during HSC maturation. (A) Expression of Hes1-GFP in endothelial cells (VC+CD45−CD43−; gate R1), pre-HSC type I (VC+CD45−CD43+; gate R2), and pre-HSC type II (VC+CD45+CD43+Sca1+; gate R3) defined by flow cytometry in the E11.5 Hes1-GFP+ AGM region. FMO GFP control (FMO control) was performed with wild-type cells. (B) Pre-HSCs type I are mainly Hes1-GFP+, whereas pre-HSCs type II reside in both the GFP− and GFP+ fraction. VC+CD45− (Pre-HSC type I) and VC+CD45+ (pre-HSC type II) cells were sorted from E10.5 and E11.5 AGM based on Hes1-GFP expression, coaggregated with OP9 cells and transplanted after culture (1 ee per recipient); n = 3. Levels of engraftment are plotted and number of repopulated vs total number of transplanted mice are shown in brackets (***P < .005, Mann-Whitney U test). (C) AGM dHSCs reside in both Hes1-GFP+ and Hes1-GFP− populations. CD45+ cells were sorted from E12.5 AGM based on Hes1-GFP expression and directly transplanted into irradiated mice (4 ee per recipient); n = 2. (D) Expression of Hes1-GFP in E14.5 fetal liver dHSCs, phenotypically defined by Lin−cKit+Sca1+CD48−CD150+. Gray histogram: Hes1-GFP− control. (E) Fetal liver (FL) HSCs reside in the GFP−/low fraction. LSK populations were sorted based on Hes1-GFP expression from E13.5 and E14.5 fetal liver and directly transplanted into irradiated mice; (0.2 ee per recipient); n = 2. LSK, Lin−Sca+cKit+; LT-HSC, long-term HSC; SSC-A, side scatter.
Notch activity decreases during HSC maturation. (A) Expression of Hes1-GFP in endothelial cells (VC+CD45−CD43−; gate R1), pre-HSC type I (VC+CD45−CD43+; gate R2), and pre-HSC type II (VC+CD45+CD43+Sca1+; gate R3) defined by flow cytometry in the E11.5 Hes1-GFP+ AGM region. FMO GFP control (FMO control) was performed with wild-type cells. (B) Pre-HSCs type I are mainly Hes1-GFP+, whereas pre-HSCs type II reside in both the GFP− and GFP+ fraction. VC+CD45− (Pre-HSC type I) and VC+CD45+ (pre-HSC type II) cells were sorted from E10.5 and E11.5 AGM based on Hes1-GFP expression, coaggregated with OP9 cells and transplanted after culture (1 ee per recipient); n = 3. Levels of engraftment are plotted and number of repopulated vs total number of transplanted mice are shown in brackets (***P < .005, Mann-Whitney U test). (C) AGM dHSCs reside in both Hes1-GFP+ and Hes1-GFP− populations. CD45+ cells were sorted from E12.5 AGM based on Hes1-GFP expression and directly transplanted into irradiated mice (4 ee per recipient); n = 2. (D) Expression of Hes1-GFP in E14.5 fetal liver dHSCs, phenotypically defined by Lin−cKit+Sca1+CD48−CD150+. Gray histogram: Hes1-GFP− control. (E) Fetal liver (FL) HSCs reside in the GFP−/low fraction. LSK populations were sorted based on Hes1-GFP expression from E13.5 and E14.5 fetal liver and directly transplanted into irradiated mice; (0.2 ee per recipient); n = 2. LSK, Lin−Sca+cKit+; LT-HSC, long-term HSC; SSC-A, side scatter.
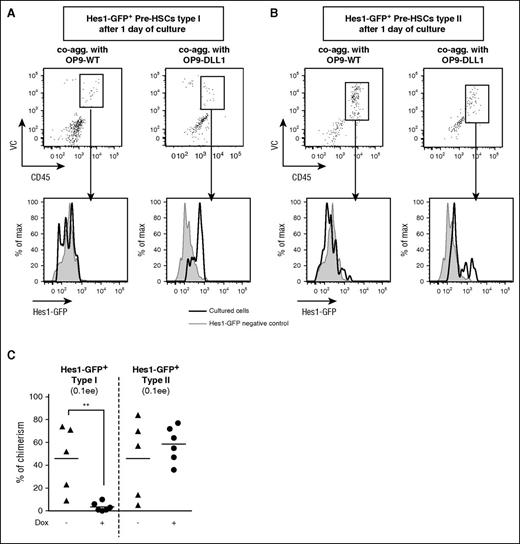
Forced Notch activity blocks pre-HSC type I maturation. (A-B) Forced activation of Notch activity elevates Hes1-GFP expression in pre-HSCs type I and type II. Hes1-GFP+ pre-HSCs type I (lin−VC+CD45−CD43+cKit+) and type II (lin−VC+CD45+Sca1+cKit+) were sorted from E11.5 AGM and coaggregated either with OP9-WT (left column) or OP9-expressing DLL1 upon doxycycline addition (right columns). After 1 day of culture, VC+CD45+ cells (dot plots) derived from pre-HSCs type I and type II were analyzed for Hes1-GFP expression (black histograms) and compared with Hes1-GFP− control cells (gray histograms). The data are representative of 2 independent experiments. (C) Forced Notch activity prevents pre-HSC type I maturation. Hes1-GFP+lin−VC+CD45−CD43+cKit+ pre-HSCs type I and Hes1-GFP+lin−VC+CD45+Sca1+cKit+ pre-HSCs type II were sorted from E11.5 AGM regions and reaggregated with OP9-Dll1. The coaggregates were cultured with cytokines with or without doxycycline for 5 days before transplantation (0.1 ee per recipient); n = 2; **P < .01, Mann-Whitney test.
Forced Notch activity blocks pre-HSC type I maturation. (A-B) Forced activation of Notch activity elevates Hes1-GFP expression in pre-HSCs type I and type II. Hes1-GFP+ pre-HSCs type I (lin−VC+CD45−CD43+cKit+) and type II (lin−VC+CD45+Sca1+cKit+) were sorted from E11.5 AGM and coaggregated either with OP9-WT (left column) or OP9-expressing DLL1 upon doxycycline addition (right columns). After 1 day of culture, VC+CD45+ cells (dot plots) derived from pre-HSCs type I and type II were analyzed for Hes1-GFP expression (black histograms) and compared with Hes1-GFP− control cells (gray histograms). The data are representative of 2 independent experiments. (C) Forced Notch activity prevents pre-HSC type I maturation. Hes1-GFP+lin−VC+CD45−CD43+cKit+ pre-HSCs type I and Hes1-GFP+lin−VC+CD45+Sca1+cKit+ pre-HSCs type II were sorted from E11.5 AGM regions and reaggregated with OP9-Dll1. The coaggregates were cultured with cytokines with or without doxycycline for 5 days before transplantation (0.1 ee per recipient); n = 2; **P < .01, Mann-Whitney test.
We then functionally tested the status of Notch activity in pre-HSCs. Pre-HSCs type I (VC+CD45−) prevail in E10.5 embryos and are gradually replaced by pre-HSCs type II (VC+CD45+) at E11.5.25 Phenotypically, we observed an increase of the GFP subset during HSC maturation (Figure 2A). However, functional analysis of sorted GFP+ and GFP− fractions by culture and transplantations showed that pre-HSCs type I (both at E10.5 and E11.5) were mainly Hes1-GFP+, whereas pre-HSCs type II resided both in GFP− and GFP+ fractions (Figure 2B).
The analysis of mature dHSCs sorted from freshly isolated AGM regions 1 day later at E12.5 showed that they also reside both in the GFP− and GFP+ fractions (Figure 2C; supplemental Figure 4A). The fetal liver HSC population, which consists of mature dHSCs29 and is defined by signaling lymphocytic activation molecule markers,60 was phenotypically predominantly Hes1-GFP+ (Figure 2D) as shown previously.61 However, functional transplantations showed that true dHSCs in E12.5 to E14.5 fetal livers reside only in Hes1-GFP− and Hes1-GFPlow fractions, with a tendency toward negative fractions (Figure 2E; supplemental Figure 4B; J.G.L., unpublished data). Thus, although Notch signaling is active in all pre-HSCs at E10.5, it is downregulated in the HSC lineage during further development.
Attenuation of Notch dependency during pre-HSC maturation
Expression analysis of Notch receptors and Hes1-GFP suggested that Notch signaling is functionally involved in pre-HSC maturation. To test this, we first blocked Notch activity by adding DAPT/γ-secretase inhibitor to AGM explant cultures and assayed the outcome of dHSCs by the long-term repopulation assay (Figure 4A). Although addition of DAPT to E10.5 AGM explant cultures almost fully blocked HSC development, the production of dHSCs by E11.5 explants was less affected as most of the recipients were repopulated, albeit at significantly lower levels compared with untreated controls (Figure 4A). Accordingly, maturation of purified pre-HSCs type II from E11.5 embryos was only partially affected by DAPT (Figure 4B). Altogether, these data suggest that although most pre-HSCs are sensitive to Notch blockade at E10.5, at later stages they do not require Notch for maturation into dHSCs. Meanwhile, in keeping with previous reports,33 production of myeloid progenitors (CFU-C) from E10.5 AGM culture was not significantly affected by DAPT treatment (Figure 4C).
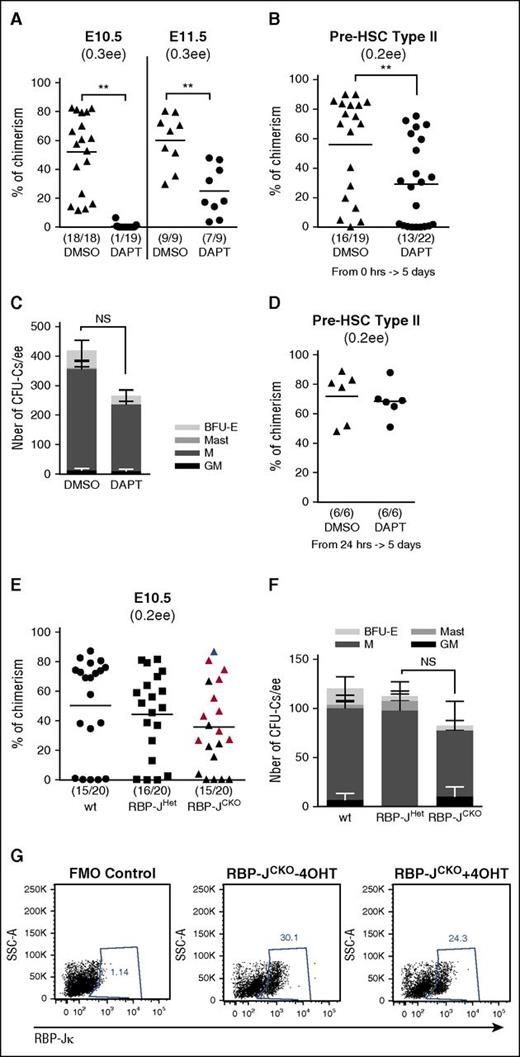
Notch signaling is required for pre-HSC development. (A) DAPT treatment completely prevents HSC development in E10.5 AGM, but has a milder effect at E11.5. E10.5 (n = 2) and E11.5 (n = 2) explants were cultured for 5 days without cytokines in the presence of dimethyl sulfoxide (DMSO) or 50 μM DAPT. At the end of the culture, the explants were dissociated and injected into irradiated mice (0.3 ee per mouse); **P < .01, Mann-Whitney test. (B) DAPT treatment disrupts pre-HSC type II maturation. E11.5-sorted VC+CD45+ cells (pre-HSC type II) were coaggregated with OP9. After 5 days of culture with cytokines in presence of DMSO or 50 μM DAPT, the reaggregates were dissociated and injected into irradiated mice (0.2 ee per mouse); n = 3, **P < .01, Mann-Whitney test. (C) DAPT treatment does not affect CFU-C development. E10.5 explants were cultured for 5 days without cytokines in presence of DMSO or 50 μM DAPT. After culture, the development of hematopoietic progenitors (CFU-C) was assessed by performing a colony-forming assay. Bars represent the average number of CFU-Cs per ee and standard errors (n = 3). (D) DAPT does not affect pre-HSC type II maturation into HSCs when added after 24 hours of culture. E11.5-sorted Hes1-GFP+ pre-HSCs type II were cultured for 24 hours prior to addition of DMSO or DAPT. After a subsequent 4 days in culture, the cells were injected into irradiated mice (0.2 ee per mouse); n = 2. (E) Conditional deletion of RBP-Jκ in the E10.5 AGM region. E10.5 RBP-JCKO, RBP-JHet, and wild-type (wt) AGM cells were dissociated, treated individually with 4-OHT for 2 hours, and then cultured as reaggregates without OP9 and without cytokines for 5 days before transplantation. The red triangles represent the recipients whose bone marrow contained RBP-JΔ/Δ dHSCs and showed T-cell phenotype; the blue triangle represents the mouse repopulated with RBP-Jflox/Δ dHSCs (normal T-cell development); black triangles represent recipients whose bone marrow was not analyzed further (0.2 ee per mouse). (F) Numbers of CFU-Cs per ee in E10.5 RBP-JCKO (CKO), RBP-JHet (Het), and WT AGM after culture (4, 3, and 4 embryos, respectively; standard errors are shown). (G) Presence of RBP-Jκ protein after induction of the deletion. E10.5 RBP-JCKO AGM was dissociated and divided into 2 parts, 1 was treated with 4-OHT and the other with methanol (4-OHT vehicle) for 2 hours at 37°C. Twenty-four hours after induction, the presence of RBP-Jκ protein was analyzed by flow cytometry. The dot plots are representative of 4 different AGMs, gated on live cells (EMA−). BFU-E, blood-forming unit-erythrocyte; GM, granulocyte-macrophage; M, macrophage; Mast, mast colonies.
Notch signaling is required for pre-HSC development. (A) DAPT treatment completely prevents HSC development in E10.5 AGM, but has a milder effect at E11.5. E10.5 (n = 2) and E11.5 (n = 2) explants were cultured for 5 days without cytokines in the presence of dimethyl sulfoxide (DMSO) or 50 μM DAPT. At the end of the culture, the explants were dissociated and injected into irradiated mice (0.3 ee per mouse); **P < .01, Mann-Whitney test. (B) DAPT treatment disrupts pre-HSC type II maturation. E11.5-sorted VC+CD45+ cells (pre-HSC type II) were coaggregated with OP9. After 5 days of culture with cytokines in presence of DMSO or 50 μM DAPT, the reaggregates were dissociated and injected into irradiated mice (0.2 ee per mouse); n = 3, **P < .01, Mann-Whitney test. (C) DAPT treatment does not affect CFU-C development. E10.5 explants were cultured for 5 days without cytokines in presence of DMSO or 50 μM DAPT. After culture, the development of hematopoietic progenitors (CFU-C) was assessed by performing a colony-forming assay. Bars represent the average number of CFU-Cs per ee and standard errors (n = 3). (D) DAPT does not affect pre-HSC type II maturation into HSCs when added after 24 hours of culture. E11.5-sorted Hes1-GFP+ pre-HSCs type II were cultured for 24 hours prior to addition of DMSO or DAPT. After a subsequent 4 days in culture, the cells were injected into irradiated mice (0.2 ee per mouse); n = 2. (E) Conditional deletion of RBP-Jκ in the E10.5 AGM region. E10.5 RBP-JCKO, RBP-JHet, and wild-type (wt) AGM cells were dissociated, treated individually with 4-OHT for 2 hours, and then cultured as reaggregates without OP9 and without cytokines for 5 days before transplantation. The red triangles represent the recipients whose bone marrow contained RBP-JΔ/Δ dHSCs and showed T-cell phenotype; the blue triangle represents the mouse repopulated with RBP-Jflox/Δ dHSCs (normal T-cell development); black triangles represent recipients whose bone marrow was not analyzed further (0.2 ee per mouse). (F) Numbers of CFU-Cs per ee in E10.5 RBP-JCKO (CKO), RBP-JHet (Het), and WT AGM after culture (4, 3, and 4 embryos, respectively; standard errors are shown). (G) Presence of RBP-Jκ protein after induction of the deletion. E10.5 RBP-JCKO AGM was dissociated and divided into 2 parts, 1 was treated with 4-OHT and the other with methanol (4-OHT vehicle) for 2 hours at 37°C. Twenty-four hours after induction, the presence of RBP-Jκ protein was analyzed by flow cytometry. The dot plots are representative of 4 different AGMs, gated on live cells (EMA−). BFU-E, blood-forming unit-erythrocyte; GM, granulocyte-macrophage; M, macrophage; Mast, mast colonies.
Because DAPT can affect other molecular pathways mediated by γ-secretase, we genetically ablated RBP-Jκ in compound Rbp-Jκflox/flox::RosaCreERT2::sGFP embryos, hereafter referred to as RBP-JCKO.37,56 Acute RBP-Jκ ablation was induced in E10.5 AGM cell suspension using 2-hour incubation with 4-OHT and after reaggregation and 5 days of culture, the generation of dHSCs and CFU-C was assessed functionally (Figure 4E-F). Concurrent Cre-mediated activation of GFP expression upon tamoxifen induction was used as a surrogate marker for Cre-mediated deletion of RBP-Jκ and for tracing donor-derived hematopoietic contribution upon transplantation (supplemental Figure 5A).
We found that CFU-Cs were not affected after deletion of RBP-Jκ but expected disruption of HSC development. To our surprise, in 9 of 10 tested recipients, transplantation of induced RBP-JCKO cells showed GFP+ long-term engraftment comparable with control transplants of wild-type and RBP-JHet cells (RBP-Jflox/+::RosaCreERT2::sGFP), although T-cell development was blocked at the double-negative stage (CD4−CD8−CD44+) as described previously for Notch deletion in adult bone marrow HSCs62 (Figure 4E; supplemental Figure 5B). At 4 months posttransplantation, bone marrow of these recipients contained mainly mutant myeloid progenitors that confirmed repopulation with RBP-JΔ/Δ HSCs (supplemental Figure 5C). Only 1 of 10 recipient transplanted with induced RBP-JCKO cells showed normal T-cell differentiation, indicating that in this case repopulation derived from HSCs that escaped deletion (supplemental Figure 5C).
Because this result is in apparent discrepancy with DAPT treatment experiments at E10.5 (Figure 4A), we analyzed the clearance of RBP-Jκ protein and found its presence at high levels 24 hours after induction of genetic ablation (Figure 4G). Only 36 hours after induction of deletion did RBP-Jκ protein completely disappear (C. Souilhol, unpublished data not shown within the figure). We reasoned that the persistence of RBP-Jκ protein during the first day of culture may ensure support of HSC development until the stage at which they become independent of Notch signaling. We tested this hypothesis by adding DAPT with a 24-hour delay and found that in this case the production of dHSCs by pre-HSC type II indeed was not affected (compare Figure 4, panels B and D). Thus, pre-HSCs become Notch independent shortly before becoming mature dHSCs. Notably, the kinetics of loss of Notch signaling corroborates this conclusion: indeed, after 24 hours in culture, pre-HSC type II, which initially express Hes1-GFP (Figure 2A), downregulated GFP (Figure 3B). Meanwhile, DAPT treatment of pre-HSC type I with 24-hour delay still negatively affected dHSC maturation (supplemental Figure 6), indicating that by that time the Notch-independent stage has not yet been reached (Hes1-GFP downregulation observed in this case [Figure 3A] is likely due to insufficient sensitivity of the reporter).
Thus, although at E10.5 pre-HSC, development depends on active Notch signaling based on the DAPT treatment experiments (Figure 4A), by E11.5 at least some pre-HSCs downregulate it (Figure 2B) and their maturation into dHSCs no longer requires Notch (Figure 4A-B,D-E).
Downregulation of Notch signaling is required for the pre-HSC type I to type II transition
Although Notch signaling is downregulated during HSC maturation and eventually becomes dispensable, it is not clear whether this downregulation is a necessary step for their development. To test this, we coaggregated Hes1-GFP+ pre-HSCs with Dox-inducible OP9-Dll1 in order to maintain Notch signaling during HSC maturation. In these experiments, we enriched pre-HSCs using positive CD43, cKit, Sca1, and lin− markers24 (J.G.L. and S.R., unpublished data not shown within the figure).
Upon doxycycline induction, Dll1 was upregulated in the inducible OP9 cell line (supplemental Figure 3A) and, accordingly, Hes1-GFP expression in pre-HSCs was maintained in culture, in contrast to control conditions with unmanipulated OP9 (Figure 3A-B). We found that such an enforced Notch activity significantly impaired maturation of pre-HSCs type I but not pre-HSCs type II (Figure 3C), suggesting that Notch downregulation is critically important for pre-HSC type I to type II transition but not thereafter.
Both Notch1 and Notch2 activity are involved in pre-HSC maturation
We have shown that both Notch1 and Notch2 are expressed in pre-HSCs (Figure 1). We therefore tested whether Notch1 and Notch2 are functionally involved in pre-HSC development using highly specific blocking antibodies, anti-NRR1 and anti-NRR2, respectively.63 Treatment with each antibody led to downregulation of Notch target genes, comparable to DAPT treatment (supplemental Figure 7A-B). Because Notch1, but not Notch2, is involved in arterial specification,64 treatment with anti-NRR1 but not anti-NRR2 downregulated Notch1 itself and CD44, both known as arterial markers65 (supplemental Figure 7C). As expected, in contrast to Notch1-mediated regulation of itself, anti-NRR2 did not reduce Notch2 protein levels (supplemental Figure 7D).
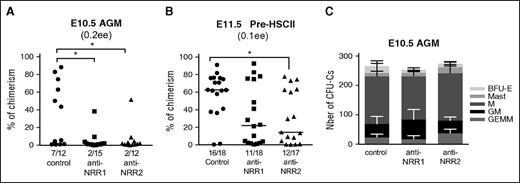
In functional experiments, E10.5 AGM (containing predominantly pre-HSC type I), or E11.5 sorted pre-HSCs enriched for pre-HSC type II, were cultured in presence of either anti-NRR1 or anti-NRR2 antibodies. Blocking of either Notch1 or Notch2 drastically suppressed HSC development in the E10.5 AGM region in agreement with DAPT treatment (compare Figures 4A and 5A). Similarly, at E11.5, maturation of sorted pre-HSCs type II was inhibited by addition of blocking antibodies, but to a lesser extent than at E10.5, in agreement with DAPT treatment (compare Figures 4B and 5B). Notably, blocking with anti-NRR2 tended to be more effective than blocking with anti-NRR1, which correlates with an increase of Notch2 and decrease of Notch1 expression in pre-HSCs type II (Figure 1B). As with DAPT treatment or after RBP-Jκ deletion, the development of CFU-Cs was not disrupted (Figure 5C). Thus, in addition to the previously reported role of Notch1, Notch2 also plays a role in HSC development.
Blocking Notch1 or Notch2 negatively affects HSC development. (A) Both Notch1 and Notch2 are involved in HSC development in the E10.5 AGM region. E10.5 AGM were cultured as reaggregates for 5 days without cytokines in the presence of anti-NRR1 (10 μg/mL) or anti-NRR2 (10 μg/mL) antibodies before transplantation into irradiated mice (0.3 ee per recipient); n = 2, *P < .05, Mann-Whitney test. (B) Blocking Notch1 or Notch2 affects pre-HSC type II maturation. E11.5 pre-HSCs type II (VC+CD45+) were coaggregated with OP9 cells with cytokines and cultured for 5 days with anti-NRR1 (10 μg/mL) or anti-NRR2 (10 μg/mL) antibodies before transplantation (0.1 ee per recipient); n = 2, *P < .05, Mann-Whitney test. (C) Blocking Notch1 or Notch2 does not disrupt CFU-C development. The development of hematopoietic progenitors in the presence of Notch-blocking antibodies after 5 days in culture, in E10.5 AGM reaggregates was assessed by CFU-C assay. The number of hematopoietic progenitors per 1 ee and standard errors are shown (n = 2). GEMM, granulocyte-erythrocyte-macrophage-megakaryocyte.
Blocking Notch1 or Notch2 negatively affects HSC development. (A) Both Notch1 and Notch2 are involved in HSC development in the E10.5 AGM region. E10.5 AGM were cultured as reaggregates for 5 days without cytokines in the presence of anti-NRR1 (10 μg/mL) or anti-NRR2 (10 μg/mL) antibodies before transplantation into irradiated mice (0.3 ee per recipient); n = 2, *P < .05, Mann-Whitney test. (B) Blocking Notch1 or Notch2 affects pre-HSC type II maturation. E11.5 pre-HSCs type II (VC+CD45+) were coaggregated with OP9 cells with cytokines and cultured for 5 days with anti-NRR1 (10 μg/mL) or anti-NRR2 (10 μg/mL) antibodies before transplantation (0.1 ee per recipient); n = 2, *P < .05, Mann-Whitney test. (C) Blocking Notch1 or Notch2 does not disrupt CFU-C development. The development of hematopoietic progenitors in the presence of Notch-blocking antibodies after 5 days in culture, in E10.5 AGM reaggregates was assessed by CFU-C assay. The number of hematopoietic progenitors per 1 ee and standard errors are shown (n = 2). GEMM, granulocyte-erythrocyte-macrophage-megakaryocyte.
Discussion
Various molecular pathways are involved in early HSC development.66 Notch signaling is critically important for early development of the hematopoietic system.67 Mutants for the main components of the Notch pathway lack hematopoietic progenitors and HSCs and showed an excess of endothelial cells.33-35,68 Several lines of evidence suggest that Notch controls the development of intraembryonic hematopoiesis by regulating essential transcription factors which are involved in the endothelial-to-hematopoietic transition.34,50 However, HSC development occurs through several sequential maturation steps, which involve a hierarchy of precursors (pre-HSCs) fully committed to hematopoietic fate. The role of Notch signaling in this hierarchical developmental process is not clear. Here we used a combination of in vitro modeling of HSC development validated by in vivo transplantations24-26 and conditional genetics to study the role of Notch signaling during the pre-HSC type I → pre-HSC type II → dHSC transitions.
Recent evidence suggests that Notch signaling is downregulated during the emergence of the hematopoietic system in the embryo.69 Notch activity is downregulated in hematopoietic clusters while maintained in the aortic endothelium, and expression of Notch target Hes1 decreases during the endothelia-to-hematopoietic transition.52,70-74 Furthermore, lowering Notch activity is required for suppression of the arterial program and acquisition of the hematopoietic fate.71,73 A recent report has also indicated that Notch signaling in HSCs is lower than in the aortic structural endothelium.59
Using Hes1-GFP reporter mice, we show functionally that although Notch signaling is active in pre-HSC type I at E10.5, it is gradually downregulated during further HSC development, initially in some pre-HSCs type II at E11.5 and subsequently in the fetal liver where the majority of transplantable HSCs become Hes1-GFP−/low.
This downregulation of Notch signaling has physiological significance because its maintenance at high levels using exogenous Dll1 is detrimental for maturation of pre-HSC type I but not at the next stage, pre-HSC type II. Furthermore, functional blockade of Notch signaling with DAPT or Notch-specific antibodies prevents pre-HSC type I development, but only moderately affects pre-HSC type II. A further 24-hour delay in DAPT treatment of pre-HSC type II completely abrogates the blocking effect, indicating complete loss of Notch dependency by the end of HSC maturation. Notch independence is a feature of adult HSCs36 and therefore our results are consistent with acquisition of the adult state by developing HSCs.
Accordingly, when we induced RBP-Jκ gene ablation, we expected to see an HSC developmental block at E10.5 and to a lesser extent at E11.5. However, we found that even at E10.5, RBP-Jκ deletion did not prevent development of HSCs, which nevertheless showed a block in T-cell differentiation typical for adult bone marrow RBP-Jκ−null HSCs.62 The generation of RBP-Jκ−mutant HSCs at E10.5 seemed to be in disparity with the blocking effects of DAPT and Notch antibodies discussed above. However, a subsequent check showed that the RBP-Jκ protein was present in cells 24 hours after induction of gene deletion. Such a delay in the loss of the RBP-Jκ protein, perhaps due to its stability, was reported previously in muscle cells.75 This must be sufficient for HSCs to pass the stage of Notch dependency in line with results of the 24-hour delay in DAPT treatment as described above.
How can these data be explained in light of a previous study reporting that the loss of RBP-Jκ in the fetal liver is detrimental for HSCs76 ? A possible explanation is that in our experiments, development in the fetal liver is circumvented by direct transplantation of AGM-derived RBP-Jκ mutant cells into the adult bone marrow environment, where, in contrast to fetal liver, HSCs may not require Notch signaling.36 A similar fetal liver stage-specific dependency was previously proposed for α4 integrin involvement in HSC development.77
To date, among the 4 known Notch receptors, only Notch1 has been shown to play a critical role in early embryonic hematopoiesis in the AGM region.33 Although previous in situ analysis suggested the presence of Notch1 but not Notch2 around the E10.5 dorsal aorta,34,72 our flow cytometry analysis showed clear expression of both Notch1 and Notch2 in the developing HSC lineage. In keeping with this, antibody blockade experiments showed that both receptors are involved in pre-HSC maturation. This contrast with Notch2 knockout studies may be explained by the fact that contrary to genetic ablation, anti-Notch2 antibody does not change the level of Notch2 protein in cells, and thus avoids triggering some compensatory mechanisms. Notably, in adults, both Notch1 and Notch2 continue to be expressed by HSCs,61,78 although signaling in HSCs is suppressed through intrinsic factors,79 possibly to avoid their potential oncogenic transformation.80 Notch1 and Notch2 involvement is mainly restricted to downstream differentiation of T cells and megakaryocyte/erythrocyte progenitors, respectively.61,62
It was previously reported that the strength of signaling through Notch2 is weaker than through Notch181,82 ; therefore, reciprocal kinetics of Notch1 and Notch2 may be responsible for attenuation of the overall Notch activity in the HSC lineage during development. The biological significance of variations in strength of Notch signaling has been reported previously. Oscillations of Hes1 regulate maintenance of neural progenitors and various Notch activity levels control the balance between quiescence and proliferation in adult neural stem cells and pancreatic endocrine progenitors.83-85 It has also been proposed that reduction in strength of Notch signaling is required for switching from the endothelial to the hematopoietic fate.52,71,74,86 Here, we specifically focused on Notch signaling during dramatic expansion of pre-HSCs in the AGM region29 which involves both maturation and slow proliferation.26 Whether downregulation of Notch signaling plays a role in setting a balance between proliferation and maturation in this process needs to be elucidated in future.
Recently, massive generation of HSCs from embryonic precursors was achieved in cocultures with engineered AKT-activated endothelial cells that strongly expressed several Notch ligands.87 Although a Notch-mediated mechanism was proposed, it required transforming growth factor β inhibition and might be explained by other than Notch-mediated mechanisms, as discussed by the authors themselves.87
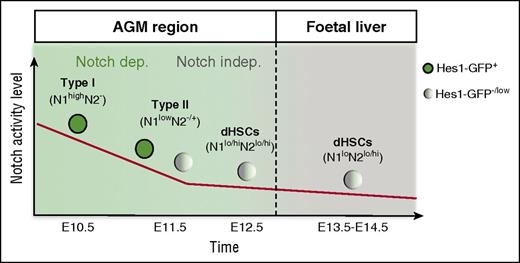
In conclusion, the HSC lineage during development in the AGM region switches from a Notch-dependent to a Notch-independent stage (Figure 6). Our results reveal a temporal window of strong Notch dependency during the pre-HSC type I to pre-HSC type II transition in the E10.5 to E11.5 AGM region (Figure 6). Once pre-HSCs pass this stage, they become significantly less dependent on Notch, which is consistent with Notch independence in adult bone marrow HSCs.36,88 Although Notch1 is dominant, both Notch1 and Notch2 have functional roles in HSC development. Careful stage-specific tuning of Notch signaling may be required when developing conditions for the generation of transplantable HSCs from pluripotent ES/induced pluripotent stem cells for therapeutic applications.
Model. In the E10.5 AGM region, Hes1-GFP is expressed in all functional pre-HSCs type I, demonstrating that the Notch pathway is activated in these cells. Notch1 is the main receptor at this stage; later on, Notch2 is upregulated during the pre-HSC type I to pre-HSC type II transition. Although both Notch1 and Notch2 are expressed in maturing pre-HSCs and dHSCs, Notch activity decreases because some pre-HSCs and dHSCs at E11.5 and E12.5, respectively, become Hes1-GFP−. Functional analysis showed that Notch activity is essential during the first steps of pre-HSC development. However, the decrease of Notch activity is accompanied by a progressive loss of Notch dependency, as some E11.5 pre-HSCs can complete development in the absence of Notch. In the fetal liver, HSCs either fully lack Notch activity or exhibit it at a low level, despite the presence of both Notch161,76 and Notch2 at their surface.
Model. In the E10.5 AGM region, Hes1-GFP is expressed in all functional pre-HSCs type I, demonstrating that the Notch pathway is activated in these cells. Notch1 is the main receptor at this stage; later on, Notch2 is upregulated during the pre-HSC type I to pre-HSC type II transition. Although both Notch1 and Notch2 are expressed in maturing pre-HSCs and dHSCs, Notch activity decreases because some pre-HSCs and dHSCs at E11.5 and E12.5, respectively, become Hes1-GFP−. Functional analysis showed that Notch activity is essential during the first steps of pre-HSC development. However, the decrease of Notch activity is accompanied by a progressive loss of Notch dependency, as some E11.5 pre-HSCs can complete development in the absence of Notch. In the fetal liver, HSCs either fully lack Notch activity or exhibit it at a low level, despite the presence of both Notch161,76 and Notch2 at their surface.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank J. Verth and C. Manson for assistance with mouse maintenance and breeding; A. Dyer for irradiations; and O. Rodriguez, F. Rossi, and C. Cryer for cell sortings. The authors thank H. Niwa for providing the plasmid pPBhCMV1-cHA-IRESVenuspA and Austin Smith for the RosaCreERT2 mice. The authors thank M. Cohen-Tannoudji, S. Gordon-Keylock, S. Lowell, K. Ottersbach, and A. Tsakiridis for helpful comments.
This work was supported by The Wellcome Trust, the People Programme (Marie Curie Actions) of the European Union’ Seventh Framework Programme FP7, Leukaemia & Lymphoma Research/Bloodwise, Biotechnology and Biological Sciences Research Council, and Medical Research Council.
Authorship
Contribution: C. Souilhol, J.G.L., S.R., D.H., F.M., H.W., A.B., A.C.M., A.B.-C., and S.Z. performed experiments; A.M., C. Souilhol, and J.G.L. designed the research and analyzed the data; C. Souilhol and J.G.L. made the figures; A.M. and C. Souilhol wrote the paper; C. Siebel provided anti-NRR blocking antibodies; H.R.M. provided anti-Notch1 antibody (clone 22E5.5; rat IgG2a); and R.K. gave the transgenic Hes1-GFP mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Medvinsky, Institute for Stem Cell Research, Medical Research Council Centre for Regenerative Medicine, University of Edinburgh, SCRM Bioquarter, 5 Little France Dr, Edinburgh E16 4UU, United Kingdom; e-mail: a.medvinsky@ed.ac.uk.
References
Author notes
C. Souilhol and J.G.L. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal