Key Points
rhIL-7 therapy was well tolerated in patients with ICL.
rhIL-7 led to increases in CD4 T cells in both peripheral blood and tissues.
Abstract
Idiopathic CD4 lymphopenia (ICL) is a rare syndrome defined by low CD4 T-cell counts (<300/µL) without evidence of HIV infection or other known cause of immunodeficiency. ICL confers an increased risk of opportunistic infections and has no established treatment. Interleukin-7 (IL-7) is fundamental for thymopoiesis, T-cell homeostasis, and survival of mature T cells, which provides a rationale for its potential use as an immunotherapeutic agent for ICL. We performed an open-label phase 1/2A dose-escalation trial of 3 subcutaneous doses of recombinant human IL-7 (rhIL-7) per week in patients with ICL who were at risk of disease progression. The primary objectives of the study were to assess safety and the immunomodulatory effects of rhIL-7 in ICL patients. Injection site reactions were the most frequently reported adverse events. One patient experienced a hypersensitivity reaction and developed non-neutralizing anti-IL-7 antibodies. Patients with autoimmune diseases that required systemic therapy at screening were excluded from the study; however, 1 participant developed systemic lupus erythematosus while on study and was excluded from further rhIL-7 dosing. Quantitatively, rhIL-7 led to an increase in the number of circulating CD4 and CD8 T cells and tissue-resident CD3 T cells in the gut mucosa and bone marrow. Functionally, these T cells were capable of producing cytokines after mitogenic stimulation. rhIL-7 was well tolerated at biologically active doses and may represent a promising therapeutic intervention in ICL. This trial was registered at www.clinicaltrials.gov as #NCT00839436.
Introduction
Idiopathic CD4 lymphopenia (ICL) is a rare syndrome characterized by consistently low CD4 T-cell counts (<300/µL) in the absence of HIV infection or other known immunodeficiency and susceptibility to opportunistic infections typically associated with AIDS.1-3 Twenty-five years have elapsed since the first reports of ICL and yet the etiology, pathogenesis, and management remain unclear. Infectious complications of ICL are largely managed and prevented with antimicrobials based on guidelines for HIV/AIDS patients; however, no proven therapies exist for ICL immunodeficiency.
Interleukin-7 (IL-7) is a cytokine produced by epithelial, stromal, and endothelial cells in the bone marrow, thymus, and lymph nodes and is essential for thymopoiesis, T-cell homeostasis, and survival.4,5 IL-7 can also enhance the killing capacity of cytotoxic CD8 T lymphocytes6 and the reactivity of antigen-specific T cells,7 thus providing the rationale for exploring a potential therapeutic role of exogenous administration of recombinant human IL-7 (rhIL-7) as treatment for ICL. In addition, previous clinical trials of rhIL-7 administration in patients with HIV8-10 and cancer,11 as well as stem cell transplant recipients,12 have established a favorable safety profile and biologic activity of this cytokine in immunocompromised patients.
We hypothesized that rhIL-7 would be safe and would lead to improved T-cell proliferation and survival in ICL patients. We report here the results of the Interleukin-7 (CYT107) Treatment of Idiopathic CD4 Lymphocytopenia: Expansion of CD4 T Cells (ICICLE) study, which was designed to evaluate the safety, pharmacokinetic, and immunologic effects of rhIL-7 in ICL patients.
Methods
Study design and objectives
ICICLE was an open-label phase 1/2A, dose-escalation study of rhIL-7 administered in patients with confirmed ICL. The study was approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, and written informed consent was obtained from all participants prior to any study procedures and in accordance with the Declaration of Helsinki. Eligible participants were adults with confirmed ICL (CD4 T-cell count <300/µL at screening and on at least 2 occasions at least 6 weeks apart in the absence of any illness, treatment, or condition accounting for CD4 lymphopenia) and increased risk for disease progression (history of opportunistic infection or CD8 cells <180/µL).3 Patients deemed to be at higher risk of untoward consequences of immune restoration as a result of ongoing uncontrolled infection, lymphoid malignancy, or autoimmune disease requiring systemic therapy were excluded. Healthy controls (HCs) were recruited under separate protocols approved by the institutional review board.
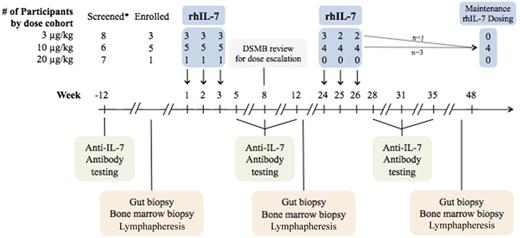
The primary objective was to evaluate the safety of a biologically active dose of rhIL-7 in the treatment of ICL patients. Secondary objectives included assessing the immunomodulatory effects of rhIL-7 on peripheral blood mononuclear cells (PBMCs) and tissue distribution of lymphocytes via optional rectosigmoid and bone marrow biopsies. The protocol was designed to study escalating doses of subcutaneous rhIL-7 administered once per week for 3 consecutive weeks at 3 µg/kg (first 3 patients), 10 µg/kg (subsequent 5 patients), and 20 µg/kg (subsequent 5 patients).
Administration of rhIL-7 (3 additional injections once per week at the same dose) was repeated at week 24, and follow-up was continued until week 48. The study team and the National Institute of Allergy and Infectious Diseases Data Safety Monitoring Board reviewed safety data until week 8 before escalation to the next dose. The protocol was later amended to allow for additional administration of rhIL-7 in patients with CD4 count remaining at <350/µL at the highest rhIL-7 dose deemed safe after safety evaluation by the DMSB.
rhIL-7 study drug
The rhIL-7 used in this study, CYT107, was supplied by Cytheris, Inc. It is a purified glycosylated 152-amino-acid rhIL-7 expressed in a Chinese hamster ovary cell line. The molecular formula for the nonglycosylated peptidic sequence is C762H1241N213O228S11.
Immunophenotyping of PBMCs
Immunophenotyping of peripheral blood drawn into tubes containing EDTA was performed according to the manufacturer’s instructions by using modified guidelines from the Centers for Disease Control and Prevention in a laboratory certified by the Clinical Laboratory Improvement Act. Cells were stained with monoclonal antibodies from BD Biosciences (San Jose, CA) and then lysed after staining with Optilyse C (Beckman Coulter, Hialeah, FL), washed twice, and re-suspended in 500 µL of phosphate-buffered saline (Cambrex, Walkersville, MD). Samples were analyzed immediately on a Becton Dickinson FACSCanto flow cytometer (BD Biosciences). FACS Diva and Flowjo software were used to analyze the data.
Statistical Methods
Median values are reported with interquartile ranges (IQRs) when appropriate. Variables at each time point were compared with week 1 (baseline) values by using the Wilcoxon matched-pairs signed rank test. Comparisons with HCs were performed by using the Mann-Whitney test. Correlations were performed by using Spearman’s coefficient. Because of the exploratory nature of the study, there was no correction for multiple comparisons, and unadjusted P values are reported. All other methods are described in detail in the supplemental Data available on the Blood Web site.
Results
Study patients
Twenty-one patients were enrolled between September 2009 and June 2013. Of these, 9 patients began study drug therapy; the demographic and baseline characteristics of these patients are provided in Table 1. Genetic screening was negative for known mutations associated with CD4 lymphopenia for each of these patients. Three patients received 3 µg/kg, 5 received 10 µg/kg, and 1 received 20 µg/kg per dose of rhIL-7 (Figure 1). Enrollment onto the study and administration of rhIL-7 were terminated in June 2013 when the study drug became unavailable. Of the 9 patients who participated, 1 patient in each of the 3 dose cohorts discontinued the study drug after 3 of 6 doses planned during the first 48 weeks: 1 patient because travel to the study site became burdensome, 1 because of a new systemic lupus erythematosus (SLE) diagnosis, and 1 because rhIL-7 was no longer available. Each participant who received the study drug was observed for a minimum of 48 weeks. Four patients, including 1 patient who initially received rhIL-7 at 3 µg/kg per dose, received additional doses of rhIL-7 at 10 µg/kg after the initial 48 weeks of rhIL-7 administration.
Patient characteristics and baseline immunologic data
| Dose of rhIL-7 (μg/kg) . | Patient ID . | Age (y)* . | Sex . | Race . | Hispanic . | Opportunistic infections . | Baseline immune profile† . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 . | CD4% . | CD8 . | CD8% . | CD4:CD8 . | B cells (CD19+) . | NK cells (CD56+CD16+) . | |||||||
| 3 | 03-001 | 51 | F | White | No | Disseminated histoplasmosis, HPV-related dysplasia | 10 | 2 | 10 | 2 | 1.0 | 603 | 140 |
| 03-004 | 23 | F | White | Yes | Severe HPV-related dysplasia | 13 | 3 | 35 | 8 | 0.4 | 684 | 104 | |
| 03-008 | 49 | F | White | No | VZV | 18 | 4 | 28 | 6 | 0.6 | 561 | 163 | |
| 10 | 10-001 | 55 | F | White | No | Cryptococcal meningitis, HPV-related dysplasia | 52 | 4 | 993 | 77 | 0.1 | 297 | 280 |
| 10-002 | 70 | M | Black | No | Kaposi’s sarcoma | 187 | 36 | 73 | 14 | 2.6 | 19 | 61 | |
| 10-003 | 56 | F | White | No | Disseminated Cryptococcus, VZV | 17 | 7 | 24 | 10 | 0.7 | 500 | 90 | |
| 10-005 | 57 | M | White | No | Recurrent oral candidiasis | 180 | 31 | 139 | 24 | 1.3 | 198 | 67 | |
| 10-006 | 34 | F | White | Yes | VZV, HPV-related dysplasia | 23 | 4 | 103 | 18 | 0.2 | 931 | 61 | |
| 20 | 20-002 | 33 | F | White | No | Severe refractory plantar warts | 15 | 3 | 25 | 5 | 0.6 | 704 | 121 |
| Dose of rhIL-7 (μg/kg) . | Patient ID . | Age (y)* . | Sex . | Race . | Hispanic . | Opportunistic infections . | Baseline immune profile† . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 . | CD4% . | CD8 . | CD8% . | CD4:CD8 . | B cells (CD19+) . | NK cells (CD56+CD16+) . | |||||||
| 3 | 03-001 | 51 | F | White | No | Disseminated histoplasmosis, HPV-related dysplasia | 10 | 2 | 10 | 2 | 1.0 | 603 | 140 |
| 03-004 | 23 | F | White | Yes | Severe HPV-related dysplasia | 13 | 3 | 35 | 8 | 0.4 | 684 | 104 | |
| 03-008 | 49 | F | White | No | VZV | 18 | 4 | 28 | 6 | 0.6 | 561 | 163 | |
| 10 | 10-001 | 55 | F | White | No | Cryptococcal meningitis, HPV-related dysplasia | 52 | 4 | 993 | 77 | 0.1 | 297 | 280 |
| 10-002 | 70 | M | Black | No | Kaposi’s sarcoma | 187 | 36 | 73 | 14 | 2.6 | 19 | 61 | |
| 10-003 | 56 | F | White | No | Disseminated Cryptococcus, VZV | 17 | 7 | 24 | 10 | 0.7 | 500 | 90 | |
| 10-005 | 57 | M | White | No | Recurrent oral candidiasis | 180 | 31 | 139 | 24 | 1.3 | 198 | 67 | |
| 10-006 | 34 | F | White | Yes | VZV, HPV-related dysplasia | 23 | 4 | 103 | 18 | 0.2 | 931 | 61 | |
| 20 | 20-002 | 33 | F | White | No | Severe refractory plantar warts | 15 | 3 | 25 | 5 | 0.6 | 704 | 121 |
F, female; HPV, human papilloma virus; M, male; NK, natural killer; VZV, varicella zoster virus (shingles).
Age at the time of enrollment.
Values reported for CD4, CD8, B cells, and NK cells are in cells per microliter. CD4% and CD8% refer to proportion of CD4+ or CD8+ cells within the CD3+ cell population. Normal ranges are CD4 T cells, 270-2110/μL; % CD4 T cells, 28-72/μL; CD8 T cells, 75-1409/μL; % CD8 T cells, 8-46/μL; CD4:CD8 ratio, 0.70-8.75; B cells (CD19+), 38-924/μL; and NK cells (CD3+CD16+CD56+), 40-740/μL.
Graphic overview of study schedule. (*) Six patients were excluded, 5 withdrew, 1 was not given rhIL-7 as a result of a study hold.
Graphic overview of study schedule. (*) Six patients were excluded, 5 withdrew, 1 was not given rhIL-7 as a result of a study hold.
Safety and pharmacokinetics
All patients who received at least 1 injection of the study drug were included in safety analysis. Supraphysiologic levels of serum IL- 7 were achieved after administration of rhIL-7 at both 3 µg/kg and 10 µg/kg (supplemental Table 1). Testing was not performed on any patient receiving 20 µg/kg.
rhIL-7 was well tolerated in patients with ICL, and there were no dose-limiting toxicities. All grade 2 adverse events (AEs) that were considered possibly, probably, or definitely related to rhIL-7 are listed in supplemental Table 2. There were no grade 3 or higher events that were considered related to the study drug. The most frequent AEs were injection site reactions (9 of 9 patients; 7 grade 1 and 2 grade 2). Five of nine patients experienced diarrhea (1 grade 1, 4 grade 2); 4 of these patients experienced 1 self-limited episode of diarrhea that resolved in less than 24 hours. The fifth patient, who had a history of gastrointestinal symptoms possibly attributable to irritable bowel syndrome experienced several episodes of diarrhea, most likely related to her underlying condition. Patients also experienced malaise (3 of 9), myalgia (4 of 9), fatigue (7 of 9), and low-grade fever (2 of 2).
One grade 2 event related to the study drug was considered to be a serious AE because of its clinical significance. Patient 10-005 developed an acute hypersensitivity reaction manifested by fever and flushing after the sixth and final injection of rhIL-7, which resolved completely within 2 days. This patient had developed non-neutralizing immunoglobulin G (IgG) and IgE antibodies against IL-7, which were no longer detectable 8 months after the event. No other patients developed binding or neutralizing antibodies to IL-7.
Patient 03-004, who did not have an autoimmune diagnosis and met all inclusion and exclusion criteria for the study at the time of screening, developed arthritis between weeks 12 and 24 of study participation and was diagnosed with SLE, which required systemic therapy. The patient did not receive further rhIL-7 and was treated with hydroxychloroquine; her arthritis improved within several months.
Although the median hemoglobin for the group (11.3 g/dL at baseline vs 11.6 g/dL at week 2 and 11.3 g/dL at week 3; supplemental Figure 1) remained unchanged after rhIL-7 therapy, hemoglobin values for 6 of 9 patients decreased to grade 1 at some point over the course of the study compared with baseline (supplemental Figure 1). Patient 03-004 was the only participant to experience grade 2 or 3 anemia (nadir, 7.6 g/dL), which was attributed to the diagnosis of SLE, and she returned to her baseline after initiation of SLE therapy.
rhIL-7 treatment led to increases in peripheral blood T cells
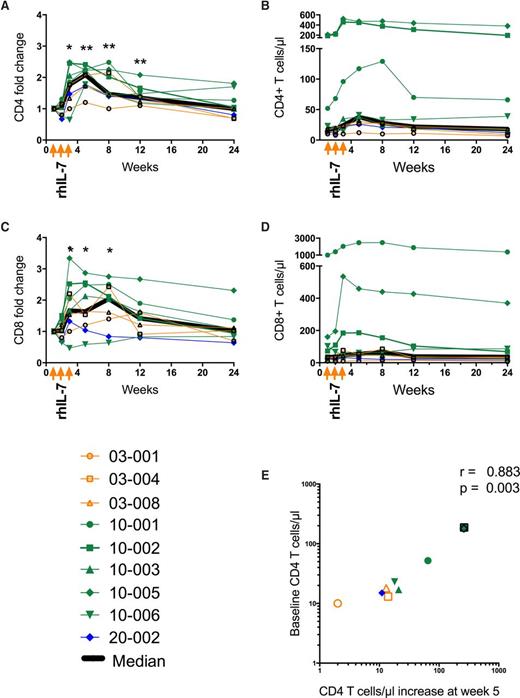
After rhIL-7 treatment, there was a significant increase in circulating CD4 T cells in patients at week 3 (fold change, 1.77; IQR, 1.165-2.255; P = .023), which peaked at week 5 (fold change, 2.08; IQR, 1.73-2.25; P = .004) and was sustained through week 12 (fold change, 1.35; IQR, 1.20-2.57; P = .004) as compared with week 1 (baseline) (Figure 2A). CD4 T-cell increases were seen in 8 of 9 study patients overall (3 of 3 in the 3-µg/kg group, 4 of 5 in the 10-µg/kg group, and 1 of 1 in the 20 µg/kg group; Figure 2B). Overall, CD8 T cells (Figure 2C-D) also increased at week 3 (fold change, 1.64; IQR, 1.12-2.5; P = .023) and week 8 (fold change, 2.04; IQR, 1.12-2.47; P = .020) compared with baseline. Two patients did not experience increases in CD8 T cells, including 1 (10-006) whose CD8 T-cell count decreased from 103/µL at week 1 to 48/µL at week 3 (Figure 2D). Increases in CD4 T cells at week 5 correlated with baseline CD4 T-cell count (r = 0.845; P = .006; Figure 2E).
Changes in circulating T cells after rhIL-7 treatment. Fold change in (A) CD4 T cells and (C) CD8 T cells in the weeks after rhIL-7 administration. Each patient’s results are depicted by a color according to dose (orange, 3 µg/kg; green, 10 µg/kg; blue, 20 µg/kg) and by a unique symbol. The bold black line represents the median value for all patients. Absolute numbers of (B) CD4 T cells and (D) CD8 T cells in each patient over the first 24 weeks of the study using the same color code and symptoms as for (A). (E) Increase in CD4 T cells at week 5 (peak CD4 T-cell count) on the x-axis compared with week 1 (baseline) CD4 T-cell count on the y-axis. Each symbol represents an individual patient using the same color scheme and symbols as in (A). *P < .05; **P < .01.
Changes in circulating T cells after rhIL-7 treatment. Fold change in (A) CD4 T cells and (C) CD8 T cells in the weeks after rhIL-7 administration. Each patient’s results are depicted by a color according to dose (orange, 3 µg/kg; green, 10 µg/kg; blue, 20 µg/kg) and by a unique symbol. The bold black line represents the median value for all patients. Absolute numbers of (B) CD4 T cells and (D) CD8 T cells in each patient over the first 24 weeks of the study using the same color code and symptoms as for (A). (E) Increase in CD4 T cells at week 5 (peak CD4 T-cell count) on the x-axis compared with week 1 (baseline) CD4 T-cell count on the y-axis. Each symbol represents an individual patient using the same color scheme and symbols as in (A). *P < .05; **P < .01.
We previously noted that patients with ICL have increased proportions of γδ T cells.13 We observed no significant changes in the proportion of γδ T cells at weeks 8, 24, or 48 (supplemental Table 3). B cells (CD19+ lymphocytes; normal range, 37-924/µL) first decreased through week 3 (294/µL at week 3 vs 561/µL at week 1; P = .359) and then increased at week 8 (702/µL; P = .008) and normalized by week 12 (561/µL; P = .07; supplemental Figure 1A). Natural killer cells (CD16+CD56+ lymphocytes; normal range, 40-740/µL) also increased, reaching statistical significance at week 5 (146/µL at week 5 vs 104/µL at week 1; P = .012; supplemental Figure 1B).
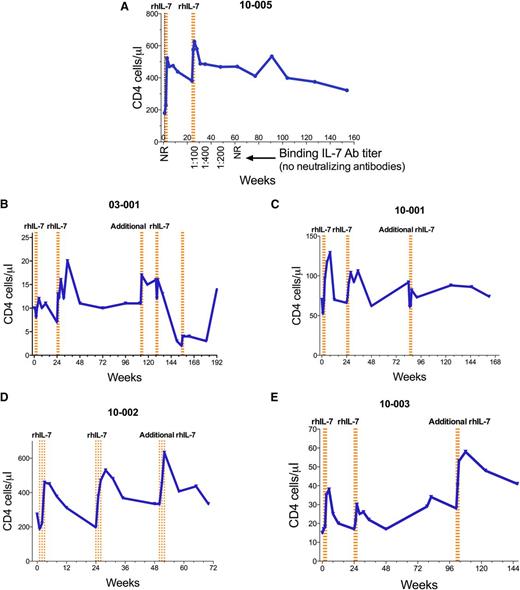
Eight patients were observed long-term beyond week 48. Patient 10-005, who experienced an acute hypersensitivity reaction after his sixth and final rhIL-7 dose, has been followed for more than 3 years since the event. Interestingly, his anti-IL-7 antibodies declined to undetectable levels within 8 months of the event, and CD4 T-cell numbers have remained above baseline and above that which would qualify for the diagnosis of ICL (300/µL) (Figure 3A). Four patients received additional doses of rhIL-7 at 10 µg/kg after completing the first 48 weeks of study.
Longitudinal CD4 T-cell counts after rhIL-7 treatment. PBMCs were assayed by flow cytometry. The blue lines in panels A-E represent CD4 T cells in the weeks after rhIL-7 initiation. rhIL-7 was administered as a series of injections once per week for 3 weeks. Each dotted vertical orange line represents a dose of rhIL-7. (A) Patient who experienced a hypersensitivity reaction after the sixth rhIL-7 dose at week 26, including the titer of non-neutralizing anti-IL-7 antibodies (Ab). (B-E) Patients received repeated rhIL-7 dosing after week 48 for CD4 T cells that remained at <350/μL.
Longitudinal CD4 T-cell counts after rhIL-7 treatment. PBMCs were assayed by flow cytometry. The blue lines in panels A-E represent CD4 T cells in the weeks after rhIL-7 initiation. rhIL-7 was administered as a series of injections once per week for 3 weeks. Each dotted vertical orange line represents a dose of rhIL-7. (A) Patient who experienced a hypersensitivity reaction after the sixth rhIL-7 dose at week 26, including the titer of non-neutralizing anti-IL-7 antibodies (Ab). (B-E) Patients received repeated rhIL-7 dosing after week 48 for CD4 T cells that remained at <350/μL.
CD4 T-cell increases may be attributable to increases in T-cell proliferation
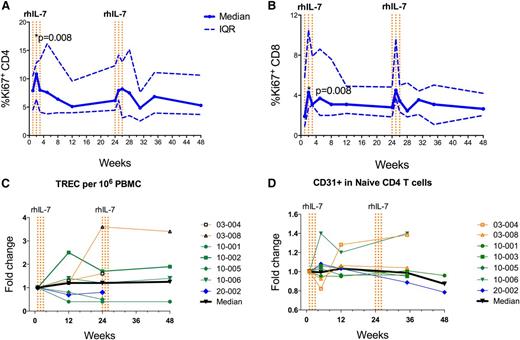
We chose IL-7 as a candidate therapy for ICL because of its ability to simultaneously stimulate thymopoiesis, increase T-cell proliferation, and prolong survival of mature T cells.14 To determine whether the T-cell responses we observed in ICL patients could be at least partially explained by an increase in T-cell cycling, we stained PBMCs from ICL patients treated with rhIL-7 at week 1 and at different time points after rhIL-7 with Ki-67, a marker of cell cycling. Soon after administration of rhIL-7, we observed a significant increase in expression of Ki-67 at week 2 in both CD4 (10.9% vs 7.9%; P = .008; Figure 4A) and CD8 (4.3% vs 1.9%; P = .008; Figure 4B) T cells, suggesting that administration of rhIL-7 increased cycling of T cells.
Mechanism of CD4 T-cell pool expansion. PBMCs were assayed by flow cytometry. (A) Proportions of CD4 and (B) CD8 T cells expressing the proliferation marker Ki-67. The solid blue line represents median values and the dotted blue lines represent the upper and lower IQRs. Data from each of the 9 patients are included at each time point. rhIL-7 was administered as a series of injections once per week for 3 weeks. Each dotted vertical orange line represents a dose of rhIL-7. (C) Fold change in T-cell receptor excision circle (TREC) content. (D) Fold change in CD31 expression in PBMCs in the weeks after initiation of rhIL-7 therapy. Each patient’s results are depicted by color and symbol as in Figure 2.
Mechanism of CD4 T-cell pool expansion. PBMCs were assayed by flow cytometry. (A) Proportions of CD4 and (B) CD8 T cells expressing the proliferation marker Ki-67. The solid blue line represents median values and the dotted blue lines represent the upper and lower IQRs. Data from each of the 9 patients are included at each time point. rhIL-7 was administered as a series of injections once per week for 3 weeks. Each dotted vertical orange line represents a dose of rhIL-7. (C) Fold change in T-cell receptor excision circle (TREC) content. (D) Fold change in CD31 expression in PBMCs in the weeks after initiation of rhIL-7 therapy. Each patient’s results are depicted by color and symbol as in Figure 2.
To assess whether enhanced thymopoiesis followed rhIL-7 treatment, we measured T-cell receptor excision circles at baseline and at weeks 12, 24, and 48 and CD31 expression on naïve CD4+ T cells at baseline and at weeks 5, 12, 35, and 48. We found no significant differences across these time points, suggesting that increased thymopoiesis did not play a major role in T-cell recovery in most patients (Figure 4C-D). Of note, two patients (03-004 and 10-006) whose T-cell receptor excision circles and CD31+ expression on naïve CD4+ T cells increased after treatment with rhIL-7 were among the youngest in the cohort (ages 23 and 34 years).
IL-7 has been shown to prolong survival of T cells by upregulating the BCL-2 family of antiapoptotic molecules.15 We measured BCL-2 expression after treatment with rhIL-7 in our cohort and found no significant changes after rhIL-7 treatment (supplemental Table 3).
Changes in T-cell phenotype after treatment with rhIL-7
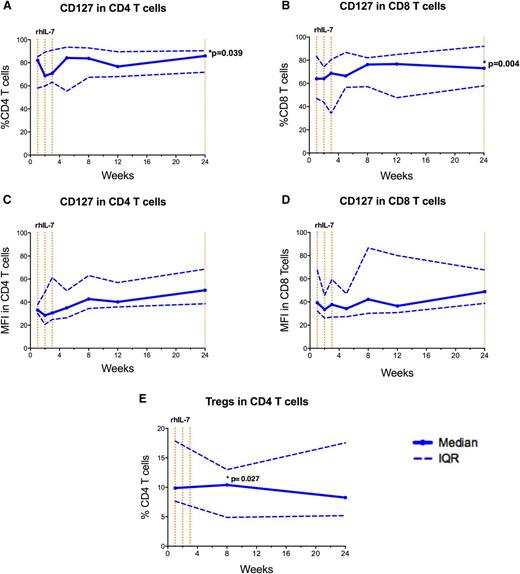
We knew from previous studies that CD127 (IL-7Rα) expression is lower in ICL patients compared with HCs3,13 and that CD127 is downregulated by IL-7 in vitro.16 After a transient decrease in the proportion of cells positive for CD127 in CD4 T cells after initiation of rhIL-7 treatment, the proportion of both CD4 and CD8 T cells expressing CD127 remained stable over the first 12 weeks of the study, and a small but statistically significant increase was observed at week 24 (CD4 T cells: 83.74% vs 74.68% at baseline [P = .039]; CD8 T cells: 73.28% vs 63.31% at baseline [P = .004]; Figure 5A-B). Mean fluorescence intensity values also trended upward and reached statistical significance at week 8 in CD4 T cells (mean fluorescence intensity: 43.3 at week 8 vs 34.3 at week 0; Figure 5C-D). Although CD127 is expressed on some differentiated T cells, expression is highest among naïve T cells. We therefore hypothesized that the delayed increases in CD127 expression we observed might be related to a preferential recovery of naïve T cells after rhIL-7 treatment, which are decreased in ICL patients.3 Previous clinical trials of rhIL-7 in other patient populations have demonstrated expansion of the naïve T-cell pool after rhIL-7 treatment.9,10,12,17 We observed no changes in the proportion of naïve CD4 T cells after rhIL-7 treatment (supplemental Table 3); however, we did see an increase in the proportion of naïve CD8 T cells (18.4% at week 1 to 27.1% at week 8; P = .039). There was also a modest decrease over time in the proportions of effector memory CD4 T cells at week 8 (26.7% at baseline to 19.4%; P = .02), at week 24 (20.7%; P = .008), and at week 48 (15.5%; P = .039) and CD8 T cells at week 8 (15.4% at week 1 to 13.7%; P = .004; supplemental Table 3).
Phenotypic T-cell changes after rhIL-7 treatment. Proportions of (A) CD4 and (B) CD8 T cells expressing CD127 (IL-7Rα). Mean fluorescence intensity (MFI) for CD127 in (C) CD4 and (D) CD8 T cells. (E) Proportion of Tregs (CD25+FoxP3+) CD4 T cells. In all five panels, the solid blue line represents median values and the dotted blue lines represent the upper and lower IQRs. Data from each of the 9 patients are included at each time point except for MFI data for CD8 T cells at week 1 for patient 10-002. rhIL-7 was administered as a series of injections once per week for 3 weeks. Each dotted vertical orange line represents a dose of rhIL-7.
Phenotypic T-cell changes after rhIL-7 treatment. Proportions of (A) CD4 and (B) CD8 T cells expressing CD127 (IL-7Rα). Mean fluorescence intensity (MFI) for CD127 in (C) CD4 and (D) CD8 T cells. (E) Proportion of Tregs (CD25+FoxP3+) CD4 T cells. In all five panels, the solid blue line represents median values and the dotted blue lines represent the upper and lower IQRs. Data from each of the 9 patients are included at each time point except for MFI data for CD8 T cells at week 1 for patient 10-002. rhIL-7 was administered as a series of injections once per week for 3 weeks. Each dotted vertical orange line represents a dose of rhIL-7.
Previous clinical studies have demonstrated that ICL patients have a high proportion of T regulatory cells (Tregs) (CD25+FoxP3+CD4+ T cells) and a high incidence of autoimmune phenomena.3 A higher proportion of Tregs at week 1 was seen in study patients compared with HCs (9.9% ICL patient vs 4.9% HC; P ≤ .001). Although the proportion of Tregs increased minimally after the initiation of rhIL-7 treatment (9.9% at week 1 vs 10.4% at week 8; P = .027; Figure 5E), it normalized by week 24 (8.3%; P = .383).
T-cell function after rhIL-7 treatment
We evaluated the potential effects of rhIL-7 on antibody-mediated immunity by assessing antibody titers for herpes simplex virus 1, herpes simplex virus 2, Epstein-Barr virus, cytomegalovirus, Helicobacter pylori, and Candida at baseline and at week 24 using the Luciferase Immunoprecipitations Systems assay. We did not observe any significant changes in antibody titers to these antigens after treatment with rhIL-7. We then assessed for changes in cytokine expression and polyfunctionality of CD4 and CD8 T cells after stimulation with selected infectious antigens (Histoplasma cell wall extract, cryptococcal capsid, tetanus toxoid, and human cytomegalovirus pp65 peptides) and mitogenic stimulation. We had adequate sample to perform this analysis on 5 patients. Although we did not detect any statistically significant increases in individual cytokine production after antigen stimulation, we did observe increases in CD4 T-cell polyfunctionality upon mitogenic stimulation in 3 of the 5 patients tested (patients 10-002, 10-005, and 10-006; Figure 6). Polyfunctionality of CD4 T cells from the remaining 2 patients tested remained unchanged (patient 10-003) or increased slightly at week 48 (patient 10-001).
Polyfunctionality of CD4 and CD8 T cells after rhIL-7 treatment. CD4 and CD8 T cells from HCs (n = 6) and study patients (n = 5) at weeks 0, 12, and 48 were stimulated with phorbol myristate acetate (PMA) and ionomycin. Stimulated cells were then analyzed by flow cytometry for the production of intracellular cytokines IL-2, tumor necrosis factor α (TNF-α), IL-17α, and interferon gamma (IFN-γ). Pie charts depict median values for HCs or individual values for each study patient at each time point, demonstrating increasing polyfunctionality (ie, increasing numbers of cytokines. The bar graphs adjacent to each pie chart demonstrate the proportion of CD4 T cells producing IL-2, TNF-α, IL-17α, and IFN-γ at the corresponding time point.
Polyfunctionality of CD4 and CD8 T cells after rhIL-7 treatment. CD4 and CD8 T cells from HCs (n = 6) and study patients (n = 5) at weeks 0, 12, and 48 were stimulated with phorbol myristate acetate (PMA) and ionomycin. Stimulated cells were then analyzed by flow cytometry for the production of intracellular cytokines IL-2, tumor necrosis factor α (TNF-α), IL-17α, and interferon gamma (IFN-γ). Pie charts depict median values for HCs or individual values for each study patient at each time point, demonstrating increasing polyfunctionality (ie, increasing numbers of cytokines. The bar graphs adjacent to each pie chart demonstrate the proportion of CD4 T cells producing IL-2, TNF-α, IL-17α, and IFN-γ at the corresponding time point.
Tissue distribution of CD3 T cells after rhIL-7 treatment
We recently reported that ICL patients are lymphopenic not only in the peripheral blood but also in the gastrointestinal mucosa.18 After observing increases in T cells in the peripheral blood following rhIL-7 treatment, we wanted to assess whether T cells were also increased in the tissues. As we found previously, immunohistochemical analysis of rectosigmoid biopsy samples of ICL patients before rhIL-7 therapy demonstrated CD3+ lymphopenia within the lamina propria compared with HCs (1.41% surface area at ICL week 0 vs 3.16% in HCs; P = .005). However, samples obtained approximately 24 weeks after initiating rhIL-7 therapy revealed an increase in CD3+ cells compared with week 0 (2.95% surface area at week 24 vs 1.41% at week 0; P = .023) which was, at that point, not different from that of HCs (2.95% surface area at week 24 vs 3.16% in HCs; P = .529; Figure 7A-B). CD4+ and CD8+ cells within the lamina propria were also increased in ICL patients after rhIL-7 treatment, but these increases did not reach statistical significance (CD4: 1.90% surface area at week 24 vs 1.1% at week 0 [P = .469]; CD8: 1.50% surface area at week 24 vs 0.90% at week 0 [P = .375]; supplemental Figure 2A-B).
Tissue distribution of CD3+ T cells after rhIL-7 treatment. Eight study patients and 17 HCs underwent flexible sigmoidoscopy with rectosigmoid biopsy for immunohistochemical analysis. (A) Representative image of CD3+ staining of the lamina propria in a study patient before and after rIL-7 treatment. The black arrows indicate areas of positive CD3 staining. (B) Percentage of lamina propria surface area staining positive for CD3 in each of the 8 patients with rectosigmoid biopsies before (week 0) and after (week 24) 3 doses of rhIL-7 treatment as well as the values for the HCs. Each patient’s results are depicted by color and symbol, as in Figure 1. HCs are depicted as black diamonds, and the straight horizontal line shows the median value for the HCs. (C) Seven study patients and 10 HCs underwent bone marrow biopsy for immunohistochemical staining for CD3. (C) Representative image of CD3+staining in the bone marrow before and after rIL-7 in a study patient. The black arrows indicate areas of positive CD3 staining. (D) Mean number of CD3+ cells per high power field for each of the 7 patients with bone marrow biopsies before and after rhIL-7 treatment. The color scheme and symbols are the same as those used in (B).
Tissue distribution of CD3+ T cells after rhIL-7 treatment. Eight study patients and 17 HCs underwent flexible sigmoidoscopy with rectosigmoid biopsy for immunohistochemical analysis. (A) Representative image of CD3+ staining of the lamina propria in a study patient before and after rIL-7 treatment. The black arrows indicate areas of positive CD3 staining. (B) Percentage of lamina propria surface area staining positive for CD3 in each of the 8 patients with rectosigmoid biopsies before (week 0) and after (week 24) 3 doses of rhIL-7 treatment as well as the values for the HCs. Each patient’s results are depicted by color and symbol, as in Figure 1. HCs are depicted as black diamonds, and the straight horizontal line shows the median value for the HCs. (C) Seven study patients and 10 HCs underwent bone marrow biopsy for immunohistochemical staining for CD3. (C) Representative image of CD3+staining in the bone marrow before and after rIL-7 in a study patient. The black arrows indicate areas of positive CD3 staining. (D) Mean number of CD3+ cells per high power field for each of the 7 patients with bone marrow biopsies before and after rhIL-7 treatment. The color scheme and symbols are the same as those used in (B).
We also investigated the impact of rhIL-7 therapy on T cells in the ICL bone marrow by using immunohistochemistry. Bone marrow CD3+ T cells were significantly lower in ICL patients before rhIL-7 therapy compared with that in HCs (13 vs 64 cells per high power field [HPF] at week 0, respectively; P ≤ .001) and increased after treatment with rhIL-7 (13 vs 34 cells per HPF; P = .031; Figure 7C-D). CD3+ T cells in the bone marrow of ICL patients remained significantly lower than those in HCs, even after rhIL-7 treatment (34 vs 64 cells per HPF, respectively; P < .001).
Changes in biomarkers after treatment with rhIL-7
Previous studies that investigated inflammatory biomarker levels in HIV-infected patients who received rhIL-7 found that restoration of gut T cells was accompanied by decreases in d-dimer levels and the monocyte activation marker sCD14, as well as phenotypic changes in peripheral blood monocytes.19 To determine whether similar changes might manifest in ICL patients, we assessed cytokine and biomarker levels at baseline and weeks 12 and 24. As expected, study patients had increased serum levels of endogenous IL-7 at baseline compared with HCs (12.70 pg/mL vs 8.62 pg/mL; P ≤ .001; Table 2). After administration of rhIL-7, endogenous IL-7 levels remained elevated at weeks 12 (P = .426) and 24 (P = 1.000; Table 2) but were not statistically different from baseline. Levels of the lymphoid trafficking chemokine SDF-1 (CXCL12) were lower in study patients at baseline compared with HCs (1813 pg/mL vs 2032 pg/mL, respectively; P < .001; Table 2) and did not change significantly at weeks 12 and 24. There were also no significant differences between levels of sCD14, d-dimer, IL-6, or C-reactive protein between week 1 and subsequent time points. We did, however, find that levels of neopterin, the proinflammatory catabolic product of guanosine triphosphate, decreased significantly at week 24 (P = .039; Table 2). Levels of sCD163, the macrophage/monocyte receptor shed after activation, increased after rhIL-7 treatment (P = .027; Table 2). Levels of the inflammatory cytokine tumor necrosis factor also slightly increased between baseline and week 12 (3.35 pg/mL at baseline vs 3.76 pg/mL at week 12; P = .020) and remained elevated at week 24 (3.72 pg/mL; P = .055; Table 2) but did not rise above levels in HCs. We concluded that administration of rhIL-7 did not significantly affect systemic inflammation or endothelial activation in ICL patients.
Soluble biomarkers before and after rhIL-7 treatment
| Biomarker . | HCs (n = 31) . | Study patients (n = 9) . | P . | ||||
|---|---|---|---|---|---|---|---|
| Week 1 . | Week 12 . | Week 24 . | HCs vs Week 1 . | Week 12 vs Week 1 . | Week 24 vs Week 1 . | ||
| IL-7 (pg/mL) | 8.62 | 12.70 | 17.69 | 20.33 | <.0001 | NS | NS |
| IL-6 (pg/mL) | 1.37 | 1.570 | 1.420 | 1.000 | NS | NS | NS |
| TNF (pg/mL) | 3.55 | 3.35 | 3.760 | 3.720 | NS | .020 | .055 |
| Eotaxin-3 (pg/mL) | 6.27 | 5.580 | 6.040 | 5.490 | NS | NS | NS |
| SDF-1 (pg/mL) | 2032 | 1813 | 1522 | 1666 | .0004 | NS | NS |
| d-dimer (mg/L) | 0.285 | 0.435 | 0.618 | 0.761 | NS | NS | NS |
| Neopterin (nmol/L) | 5.780 | 4.080 | 4.260 | 3.010 | .016 | NS | .039 |
| CRP (mg/L) | 1.700 | 2.170 | 1.488 | 1.411 | NS | NS | NS |
| sCD14 (μg/mL) | 1.214 | 1.447 | 1.372 | 1.526 | NS | NS | NS |
| sCD163 (pg/mL) | 365.8 | 153.0 | 336.9 | 281.9 | .205 | .027 | NS |
| Biomarker . | HCs (n = 31) . | Study patients (n = 9) . | P . | ||||
|---|---|---|---|---|---|---|---|
| Week 1 . | Week 12 . | Week 24 . | HCs vs Week 1 . | Week 12 vs Week 1 . | Week 24 vs Week 1 . | ||
| IL-7 (pg/mL) | 8.62 | 12.70 | 17.69 | 20.33 | <.0001 | NS | NS |
| IL-6 (pg/mL) | 1.37 | 1.570 | 1.420 | 1.000 | NS | NS | NS |
| TNF (pg/mL) | 3.55 | 3.35 | 3.760 | 3.720 | NS | .020 | .055 |
| Eotaxin-3 (pg/mL) | 6.27 | 5.580 | 6.040 | 5.490 | NS | NS | NS |
| SDF-1 (pg/mL) | 2032 | 1813 | 1522 | 1666 | .0004 | NS | NS |
| d-dimer (mg/L) | 0.285 | 0.435 | 0.618 | 0.761 | NS | NS | NS |
| Neopterin (nmol/L) | 5.780 | 4.080 | 4.260 | 3.010 | .016 | NS | .039 |
| CRP (mg/L) | 1.700 | 2.170 | 1.488 | 1.411 | NS | NS | NS |
| sCD14 (μg/mL) | 1.214 | 1.447 | 1.372 | 1.526 | NS | NS | NS |
| sCD163 (pg/mL) | 365.8 | 153.0 | 336.9 | 281.9 | .205 | .027 | NS |
CRP, C-reactive protein; NS, not significant.
Similar to what others described in HIV-infected patients after rhIL-7 treatment,19 we observed a small increase in the expression of the monocyte chemokine receptor CCR2 after initiating rhIL-7 therapy (92.2% at week 24 vs 85.7% at week 1; P = .008) with a decrease in the chemokine receptor CX3CR1 (6.7% at week 24 vs 12.6% at week 1; P = .016; supplemental Table 4). We also detected a decrease in the proportion of CD14dimCD16+ (patrolling) monocytes at week 24 (P = .039; supplemental Table 4).
Discussion
There are currently no approved therapies for patients with ICL and yet these patients are at high risk for opportunistic infections2,3,20-35 and viral-associated dysplasia and malignancies.36-42 IL-7 plays an essential role in T-cell homeostasis and T-cell survival and has been found to be well tolerated in humans with various lymphopenic conditions including HIV8-10 and cancer,11 as well as in stem cell transplant recipients.12 These previous studies showed that administration of IL-7 expands CD4 and CD8 T cells, decreases Tregs, and can help restore CD4 T cells in the gut mucosa of lymphopenic HIV patients as a result of increased T-cell homing and possibly local expansion. Because the main immunologic defect in ICL is severe T-cell lymphopenia, rhIL-7 was a rational choice for therapeutic intervention.
Although we chose IL-7 because of its ability to simultaneously stimulate thymopoiesis,14,43 increase T-cell proliferation,44,45 and prolong survival of mature T cells,46 it was not clear before this study which if any of these functions of IL-7 would be effective in T-cell recovery in ICL. ICL T cells express high levels of activation and proliferation markers3 and, as recently reported, are prematurely senescent.47 Furthermore, CD4 and CD8 T cells from ICL patients have blunted responses to in vitro stimulation with IL-7 compared with controls,13 which suggests an underutilization of existing cytokine. Our data supported an important role of T-cell proliferation in the T-cell expansion seen after rhIL-7 treatment in ICL patients. Despite elevated serum levels of IL-7 at baseline and decreased responsiveness to IL-7 in vitro,13 the results of our study demonstrate that rhIL-7 is biologically active in ICL patients, even at low doses. CD4 T-cell increases were observed in 8 of 9 study patients, were substantial in several patients with moderate lymphopenia, and were sustained through week 12 in peripheral blood. However, CD4 T-cell count increases were modest in patients with severe CD4 lymphopenia. We did not find significant differences in levels of biomarkers of systemic inflammation or endothelial activation between HCs and ICL patients at baseline. Administration of rhIL-7 did not significantly affect systemic inflammation or endothelial activation in ICL patients and did not lead to changes in activation or cycling of CD4 T cells. Although our data did not suggest a dose-dependent response to rhIL-7 treatment, the limited number of patients evaluated in each dose cohort (1 at the 20-μg/kg dose) and the heterogeneity of the ICL population make it difficult to conclude definitively that there would not be a relationship in a larger study. Expansion of T cells was also observed in tissue sampled 24 weeks after rhIL-7 therapy, which showed that expanded T cells had sufficient homing potential and that they populated effector sites.
rhIL-7 therapy was well tolerated, even when patients received repeated doses. The patient who developed an acute hypersensitivity reaction after his sixth dose of rhIL-7 was the only patient to develop anti-IL-7 antibodies (non-neutralizing). The CD4 T-cell count had increased 3.5-fold and remained above the threshold for ICL diagnosis (300/µL) 3 years later. Studies in murine models have demonstrated that anti-cytokine antibodies can actually enhance and prolong the function of cytokines such as IL-7 through the formation of complexes containing anti-IL-7 and IL-7.48 It is therefore possible that this patient’s anti-IL-7 antibodies contributed to his remarkable CD4 T-cell response to rhIL-7.
Although the small sample size and clinically stable patients enrolled onto the study did not allow determination of potential clinical benefit, the decrease in Tregs, increase in CD127-expressing T cells, and decrease in effector memory cells, along with a more polyfunctional profile of CD4 T cells after therapy in some patients, seem promising for possible improvement of T-cell function that should be addressed in future studies. It is important to note that although this study excluded ICL patients with poorly controlled opportunistic infections because of safety concerns, rhIL-7 has been enlisted for compassionate use in 3 cases of progressive multifocal leukoencephalopathy with apparent success.49-51
In conclusion, the results of this study demonstrate that rhIL-7 is safe and well tolerated in ICL and can induce cycling and expansion of T cells in peripheral blood and tissues in even the most severely lymphopenic patients. The results of this study provide the basis for further evaluation of clinical efficacy of rhIL-7 therapy in treatment of ICL patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the study participants, referring physicians, and the clinical staff of Outpatient Clinic 8 at the National Institutes of Health and acknowledge Catherine Rehm and Sara Jones for logistical support, Sonya Krishnan for sample processing, Maura Manion and Elizabeth Richards for performing additional assays of T-cell function, and the National Institute of Allergy and Infectious Diseases Data Safety Monitoring Board and Institutional Review Board.
This work was supported in whole or in part by the intramural research programs of the National Institute of Allergy and Infectious Diseases, the National Institute of Dental and Craniofacial Research, an intramural Bench-to-Bedside grant from the National Institutes of Health (NIH), and by federal funding from the National Cancer Institute, NIH, under Contract No. HHSN261200800001E.
Authorship
Contribution: I.S. and B.O.P. developed the hypotheses and study design; I.S., V.S., B.O.P., G.R., A.F.F., J.M., and A.P. provided clinical care for the patients; G.R., V.S., and B.O.P. recruited and screened study participants and collected and reported safety data; S.B.K., J.H., W.L.T., S.M.R.J., J.A., and J.S.B. performed peripheral blood mononuclear cell phenotyping and analyzed and interpreted data; A.R. performed serum and plasma cytokine measurements; P.D.B. measured antibody titers to infectious pathogens; V.N. performed T-cell receptor excision circle measurements; S.M.R.J. performed mitogen/antigen stimulation assays; M.D.Y. performed gastrointestinal biopsies; D.R.M. and J.D.E. analyzed the gastrointestinal biopsy material; L.O. and I.M. analyzed bone marrow biopsy material; V.S., I.S., R.D., S.B.K., A.P.-D., J.S.B., S.M.R.J., L.O., V.N., P.D.B., D.R.M., and J.D.E. analyzed data; T.C. contributed to pharmacokinetic data acquisition, analysis, and interpretation; R.D. assisted with study design and performed statistical analyses; V.S., I.S., and A.P.-D. wrote the manuscript; and each author reviewed the manuscript prior to final submission.
Conflict-of-interest disclosure: T.C. was an employee and shareholder of Cytheris. B.O.P. contributed to this work while employed by the National Institutes of Health. The remaining authors declare no competing financial interests.
Correspondence: Virginia Sheikh, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Dr, Building 10, Room 11C422, Bethesda, MD 20892; e-mail: sheikhv@niaid.nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal