Key Points
Benefit from panobinostat-dexamethasone-bortezomib was greatest in patients who received ≥2 prior regimens including bortezomib and IMiDs.
Abstract
Panobinostat is a potent pan-deacetylase inhibitor that affects the growth and survival of multiple myeloma (MM) cells through alteration of epigenetic mechanisms and protein metabolism. Panobinostat plus bortezomib and dexamethasone (PAN-BTZ-Dex) led to a significant increase in progression-free survival (PFS) vs placebo plus bortezomib and dexamethasone (Pbo-BTZ-Dex) in patients with relapsed or relapsed and refractory MM in the phase 3 PANORAMA 1 trial. This subgroup analysis evaluated outcomes in patients in the PANORAMA 1 trial based on prior treatment: a prior immunomodulatory drug (IMiD; n = 485), prior bortezomib plus an IMiD (n = 193), and ≥2 prior regimens including bortezomib and an IMiD (n = 147). Median PFS with PAN-BTZ-Dex vs Pbo-BTZ-Dex across subgroups was as follows: prior IMiD (12.3 vs 7.4 months; hazard ratio [HR], 0.54; 95% confidence interval [CI], 0.43-0.68), prior bortezomib plus IMiD (10.6 vs 5.8 months; HR, 0.52; 95% CI, 0.36-0.76), and ≥2 prior regimens including bortezomib and an IMiD (12.5 vs 4.7 months; HR, 0.47; 95% CI, 0.31-0.72). Common grade 3/4 adverse events and laboratory abnormalities in patients who received PAN-BTZ-Dex across the prior treatment groups included thrombocytopenia, lymphopenia, neutropenia, diarrhea, and asthenia/fatigue. Incidence of on-treatment deaths among patients who received prior bortezomib and an IMiD (regardless of number of prior regimens) was similar between treatment arms. This analysis demonstrated a clear PFS benefit of 7.8 months with PAN-BTZ-Dex among patients who received ≥2 prior regimens including bortezomib and an IMiD, a population with limited treatment options and poorer prognosis. This trial was registered at www.clinicaltrials.gov as #NCT01023308.
Introduction
Over the past decade, there have been significant advancements in the development of treatments for patients with multiple myeloma (MM), which have led to clear improvements in overall survival (OS).1,2 These advancements have focused primarily on 2 drug classes: proteasome inhibitors (bortezomib and carfilzomib) and immunomodulatory drugs (IMiDs; thalidomide, lenalidomide, and pomalidomide). Although these agents have contributed substantially to improved outcomes for patients with MM, current therapies are not curative, and an unmet medical need exists in patients who progress following treatment.3 Thus, new agents with novel mechanisms of action are urgently needed for patients who progress following treatment with proteasome inhibitors and/or IMiDs.
Panobinostat is a potent pan-deacetylase inhibitor that inhibits key aberrations in MM cell biology, including epigenetics and protein metabolism.4,5 A randomized, double-blind, phase 3 study (PANORAMA 1) of panobinostat plus bortezomib and dexamethasone (PAN-BTZ-Dex; N = 387) vs placebo plus bortezomib and dexamethasone (Pbo-BTZ-Dex; N = 381) in patients with relapsed or relapsed and refractory MM demonstrated a significant and clinically meaningful increase in median progression-free survival (PFS) of ∼4 months for patients in the PAN-BTZ-Dex arm compared with patients in the Pbo-BTZ-Dex arm (12.0 vs 8.1 months; P < .0001). In addition, the rate of high-quality responses (near-complete response [nCR]/complete response [CR]) was higher in the PAN-BTZ-Dex arm than in the Pbo-BTZ-Dex arm (28% vs 16%; P = .00006). Grade 3/4 adverse events (AEs) and hematologic laboratory abnormalities were higher among patients in the PAN-BTZ-Dex arm.6 The most common grade 3/4 AEs and hematologic laboratory abnormalities among patients who received panobinostat were thrombocytopenia (67%), lymphopenia (53%), diarrhea (26%), and fatigue/asthenia (24%). The percentage of on-treatment deaths was higher in the PAN-BTZ-Dex arm (n = 30; 8%) than in the Pbo-BTZ-Dex arm (n = 18; 5%).
The results from PANORAMA 1 clearly demonstrate that panobinostat increases the median PFS in patients with relapsed or relapsed and refractory MM when added to bortezomib and dexamethasone. However, patients who progress following treatment with bortezomib and IMiDs have a poor prognosis; therefore, an analysis of outcomes based on prior treatment was conducted. Here, we present the results among patients who received prior IMiD, prior bortezomib and IMiD, and ≥2 prior regimens including bortezomib and an IMiD to determine outcomes in these patient populations with a clear unmet need.
Materials and methods
Study design and treatment schedule
The design and treatment schedule, including the CONSORT diagram, for the PANORAMA 1 trial was described previously.6 In brief, PANORAMA 1 is a multicenter, double-blind, phase 3 study evaluating PAN-BTZ-Dex vs Pbo-BTZ-Dex in patients with relapsed or relapsed and refractory MM. Patients were randomized at a 1:1 ratio and stratified according to prior receipt of bortezomib and by the number of prior treatment lines (1 vs 2 or 3). The primary end point of the trial was PFS as per investigator’s assessment. The key secondary end point was OS. Other end points included overall response rate (ORR; ≥ partial response [PR]), rate of ≥ nCR, duration of response, time to treatment response, time to treatment progression, and safety. Response was assessed using the modified European Group for Blood and Marrow Transplantation criteria.7,8 AEs were assessed as per the Common Toxicity Criteria for Adverse Events version 3.0. The treatment-free interval (TFI) was also determined by partitioning PFS into treatment period and treatment-free period for the overall population and for each prior treatment subgroup.
In PANORAMA 1, patients received a maximum of 12 cycles of treatment across 2 treatment phases. Treatment phase 1 consisted of eight 3-week cycles of panobinostat (20 mg, orally) or placebo administered 3 times per week for 2 out of 3 weeks. Bortezomib (1.3 mg/m2, intravenously) was administered twice weekly with dexamethasone (20 mg, orally) administered on the days of and after bortezomib administration. Patients who demonstrated clinical benefit, defined as ≥ no change in disease findings per modified European Group for Blood and Marrow Transplantation criteria by cycle 8, could proceed to treatment phase 2. Treatment phase 2 consisted of four 6-week cycles during which panobinostat was administered on the same schedule, whereas bortezomib was reduced to a once-weekly schedule, with dexamethasone again administered on the day of and after bortezomib.
This trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice. Written informed consent was obtained from all patients, and the protocol was approved by institutional review boards or ethics committees at all participating institutions.
Patients
Detailed patient eligibility criteria for the PANORAMA 1 study were described previously.6 Briefly, patients with relapsed or relapsed and refractory MM with measurable disease who received 1 to 3 prior lines of therapy were eligible. Patients with bortezomib-refractory or primary refractory MM were not eligible. For this analysis, patient groups from the PANORAMA 1 study population were identified based on prior treatment with bortezomib or an IMiD (lenalidomide and/or thalidomide) and the number of prior regimens (≥2). Three patient groups were identified and analyzed for clinical efficacy and safety outcomes: (1) patients who received prior IMiD, (2) patients who received prior bortezomib and IMiD, and (3) patients who received ≥2 prior regimens including bortezomib and an IMiD.
Statistical analyses
Statistical analyses were preplanned for the prior IMiD and prior bortezomib and IMiD groups. A smaller subset analysis was performed for the group of patients who received ≥2 prior regimens including bortezomib and an IMiD. Kaplan-Meier method was used for PFS (the primary end point from the PANORAMA 1 trial) for all groups. Comparative analyses were performed between the 2 treatment arms in all groups for efficacy and safety end points. Calculation of TFI took into consideration that PFS consists of 2 distinct periods: the treatment period and the TFI.9 For the treatment period, if the PFS event or censoring occurred on or after the end of treatment, then the treatment period is from initiation through end of treatment. However, if the PFS event or censoring occurred before the end of treatment, then the treatment period is from initiation of treatment through the date of the PFS event or censoring. The TFI was calculated from the end of the treatment period as described above through the PFS event or censoring. The TFI type (event or censored) will be classified as per the PFS and will be censored at day 1 if the PFS is censored before end of treatment. The mean of the TFI was calculated as the difference between mean PFS and mean treatment period. Treatment duration and PFS were also summarized using medians for completeness.
All authors had access to the primary clinical trial data.
Results
Patient characteristics
Baseline patient characteristics and treatment history are shown in Table 1. A total of 485 patients enrolled into the PANORAMA 1 trial received prior IMiD (63% of total population: PAN-BTZ-Dex, n = 245; Pbo-BTZ-Dex, n = 240), 193 received prior bortezomib plus IMiD (25% of total population: PAN-BTZ-Dex, n = 94; Pbo-BTZ-Dex, n = 99), and 147 received ≥2 prior regimens including bortezomib and an IMiD (19% of total population: PAN-BTZ-Dex, n = 73; Pbo-BTZ-Dex, n = 74). Overall, patient characteristics appeared fairly well balanced between treatment arms (PAN-BTZ-Dex vs Pbo-BTZ-Dex) within each prior treatment group (Table 1). For example, the median age and proportion of patients with renal impairment (creatinine clearance rate ≥60-90 mL/min) were consistent across all prior treatment groups. Notable differences (>10%) between treatment arms were observed in a few instances. Differences in baseline Eastern Cooperative Oncology Group (ECOG) performance status were noted in the prior bortezomib plus IMiD group (ECOG performance status ≥1: PAN-BTZ-Dex, 40%; Pbo-BTZ-Dex, 59%) and in the group with ≥2 prior regimens including bortezomib and an IMiD (ECOG performance status ≥1: PAN-BTZ-Dex, 44%; Pbo-BTZ-Dex, 61%). There was also a higher percentage of patients with relapsed and refractory disease in the Pbo-BTZ-Dex arm of the group with ≥2 prior regimens including bortezomib and an IMiD (PAN-BTZ-Dex, 47%; Pbo-BTZ-Dex, 58%).
Patient characteristics and demographics based on prior treatment
| . | Prior IMiD . | Prior BTZ + IMiD . | ≥2 prior regimens including BTZ and an IMiD . | |||
|---|---|---|---|---|---|---|
| PAN-BTZ-Dex (n = 245) . | Pbo-BTZ-Dex (n = 240) . | PAN-BTZ-Dex (n = 94) . | Pbo-BTZ-Dex (n = 99) . | PAN-BTZ-Dex (n = 73) . | Pbo-BTZ-Dex (n = 74) . | |
| Age, median (range), y | 62 (28-82) | 62 (32-81) | 60 (28-79) | 61 (32-77) | 61 (33-79) | 61 (32-77) |
| Time since diagnosis, median (range), mo | 37.7 (4.4-308.1) | 39.9 (2.4-174.9) | 45.1 (8.7-308.1) | 40.2 (11.9-164.8) | 52.9 (10.3-308.1) | 46.3 (11.9-164.8) |
| Sex, n (%) | ||||||
| Male | 125 (51) | 130 (54) | 52 (55) | 49 (49) | 41 (56) | 33 (45) |
| Female | 120 (49) | 110 (46) | 42 (45) | 50 (51) | 32 (44) | 41 (55) |
| ECOG performance status, n (%) | ||||||
| 0 | 121 (49) | 96 (40) | 56 (60) | 38 (38) | 41 (56) | 26 (35) |
| ≥1 | 124 (51) | 144 (60) | 38 (40) | 58 (59) | 32 (44) | 45 (61) |
| ISS stage, n (%) | ||||||
| I | 106 (43) | 99 (41) | 40 (43) | 35 (35) | 31 (43) | 25 (34) |
| II | 60 (25) | 55 (23) | 21 (22) | 19 (19) | 17 (23) | 15 (20) |
| III | 46 (19) | 51 (21) | 19 (20) | 24 (24) | 15 (21) | 19 (26) |
| Not assessed | 33 (14) | 35 (15) | 14 (15) | 21 (21) | 10 (14) | 15 (20) |
| Renal function, n (%) | ||||||
| CCr ≥90 mL/min | 80 (33) | 82 (34) | 35 (37) | 34 (34) | 26 (36) | 25 (34) |
| CCr ≥60-90 mL/min | 164 (67) | 156 (65) | 59 (63) | 63 (64) | 47 (64) | 48 (65) |
| Missing | 1 (0.4) | 2 (1) | 0 | 2 (2) | 0 | 1 (1) |
| MM disease characteristics, n (%) | ||||||
| Relapsed | 127 (52) | 118 (49) | 48 (51) | 44 (44) | 39 (53) | 30 (41) |
| Relapsed and refractory | 115 (47) | 119 (50) | 46 (49) | 53 (54) | 34 (47) | 43 (58) |
| Prior lines of therapy, median (range), n | 2 (1-4) | 2 (1-3) | 2 (1-4) | 2 (1-3) | 3 (2-4) | 3 (2-3) |
| Prior ASCT, n (%) | 140 (57) | 140 (58) | 68 (72) | 69 (70) | 54 (74) | 47 (64) |
| Prior therapies, n (%) | ||||||
| Bortezomib | 94 (38) | 99 (41) | 94 (100) | 99 (100) | 73 (100) | 74 (100) |
| Lenalidomide | 72 (29) | 85 (35) | 34 (36) | 45 (46) | 28 (38) | 37 (50) |
| Thalidomide | 205 (84) | 188 (78) | 78 (83) | 69 (70) | 63 (86) | 50 (68) |
| Bortezomib + lenalidomide | 34 (14) | 45 (19) | 34 (36) | 45 (46) | 28 (38) | 37 (50) |
| Bortezomib + dexamethasone | 89 (36) | 94 (39) | 89 (95) | 94 (95) | 69 (95) | 74 (100) |
| . | Prior IMiD . | Prior BTZ + IMiD . | ≥2 prior regimens including BTZ and an IMiD . | |||
|---|---|---|---|---|---|---|
| PAN-BTZ-Dex (n = 245) . | Pbo-BTZ-Dex (n = 240) . | PAN-BTZ-Dex (n = 94) . | Pbo-BTZ-Dex (n = 99) . | PAN-BTZ-Dex (n = 73) . | Pbo-BTZ-Dex (n = 74) . | |
| Age, median (range), y | 62 (28-82) | 62 (32-81) | 60 (28-79) | 61 (32-77) | 61 (33-79) | 61 (32-77) |
| Time since diagnosis, median (range), mo | 37.7 (4.4-308.1) | 39.9 (2.4-174.9) | 45.1 (8.7-308.1) | 40.2 (11.9-164.8) | 52.9 (10.3-308.1) | 46.3 (11.9-164.8) |
| Sex, n (%) | ||||||
| Male | 125 (51) | 130 (54) | 52 (55) | 49 (49) | 41 (56) | 33 (45) |
| Female | 120 (49) | 110 (46) | 42 (45) | 50 (51) | 32 (44) | 41 (55) |
| ECOG performance status, n (%) | ||||||
| 0 | 121 (49) | 96 (40) | 56 (60) | 38 (38) | 41 (56) | 26 (35) |
| ≥1 | 124 (51) | 144 (60) | 38 (40) | 58 (59) | 32 (44) | 45 (61) |
| ISS stage, n (%) | ||||||
| I | 106 (43) | 99 (41) | 40 (43) | 35 (35) | 31 (43) | 25 (34) |
| II | 60 (25) | 55 (23) | 21 (22) | 19 (19) | 17 (23) | 15 (20) |
| III | 46 (19) | 51 (21) | 19 (20) | 24 (24) | 15 (21) | 19 (26) |
| Not assessed | 33 (14) | 35 (15) | 14 (15) | 21 (21) | 10 (14) | 15 (20) |
| Renal function, n (%) | ||||||
| CCr ≥90 mL/min | 80 (33) | 82 (34) | 35 (37) | 34 (34) | 26 (36) | 25 (34) |
| CCr ≥60-90 mL/min | 164 (67) | 156 (65) | 59 (63) | 63 (64) | 47 (64) | 48 (65) |
| Missing | 1 (0.4) | 2 (1) | 0 | 2 (2) | 0 | 1 (1) |
| MM disease characteristics, n (%) | ||||||
| Relapsed | 127 (52) | 118 (49) | 48 (51) | 44 (44) | 39 (53) | 30 (41) |
| Relapsed and refractory | 115 (47) | 119 (50) | 46 (49) | 53 (54) | 34 (47) | 43 (58) |
| Prior lines of therapy, median (range), n | 2 (1-4) | 2 (1-3) | 2 (1-4) | 2 (1-3) | 3 (2-4) | 3 (2-3) |
| Prior ASCT, n (%) | 140 (57) | 140 (58) | 68 (72) | 69 (70) | 54 (74) | 47 (64) |
| Prior therapies, n (%) | ||||||
| Bortezomib | 94 (38) | 99 (41) | 94 (100) | 99 (100) | 73 (100) | 74 (100) |
| Lenalidomide | 72 (29) | 85 (35) | 34 (36) | 45 (46) | 28 (38) | 37 (50) |
| Thalidomide | 205 (84) | 188 (78) | 78 (83) | 69 (70) | 63 (86) | 50 (68) |
| Bortezomib + lenalidomide | 34 (14) | 45 (19) | 34 (36) | 45 (46) | 28 (38) | 37 (50) |
| Bortezomib + dexamethasone | 89 (36) | 94 (39) | 89 (95) | 94 (95) | 69 (95) | 74 (100) |
ASCT, autologous stem cell transplant; CCr, creatinine clearance rate; ISS, International Staging System.
As expected, patients in the groups with prior bortezomib plus an IMiD and ≥2 prior regimens including bortezomib and an IMiD were more heavily pretreated and demonstrated a longer time since diagnosis than did the patients in the prior IMiD group (Table 1). Notably, in the group with ≥2 prior regimens including bortezomib and an IMiD, the median prior lines of therapy and percentage of patients who received previous autologous stem cell transplant, bortezomib plus lenalidomide, or bortezomib plus dexamethasone were all higher compared with the other subgroups.
Efficacy
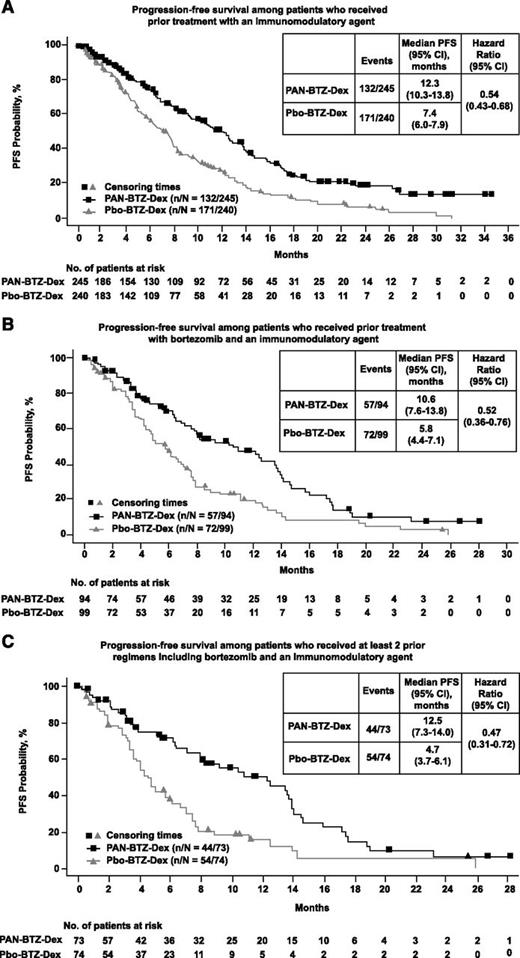
An increase in median PFS among patients in the PAN-BTZ-Dex arm was observed across all prior treatment groups (Figure 1). In patients who received prior IMiD, the median PFS benefit for the PAN-BTZ-Dex arm was 4.9 months: PAN-BTZ-Dex, 12.3 months (95% confidence interval [CI], 10.3-13.8); Pbo-BTZ-Dex, 7.4 months (95% CI, 6.0-7.9); hazard ratio (HR), 0.54 (95% CI, 0.43-0.68; Figure 1A). A similar difference in median PFS was observed in patients with prior bortezomib plus IMiD (4.8 months): PAN-BTZ-Dex, 10.6 months (95% CI, 7.6-13.8); Pbo-BTZ-Dex, 5.8 months (95% CI, 4.4-7.1); HR, 0.52 (95% CI, 0.36-0.76; Figure 1B). Among the 3 groups, the greatest difference in median PFS between the PAN-BTZ-Dex and Pbo-BTZ-Dex arms was observed in patients who received ≥2 prior regimens including bortezomib and an IMiD (Figure 1C). The difference in median PFS in these patients was 7.8 months: PAN-BTZ-Dex, 12.5 months (95% CI, 7.3-14.0); Pbo-BTZ-Dex, 4.7 months (95% CI, 3.7-6.1); HR, 0.47 (95% CI, 0.31-0.72). In patients with no prior exposure to bortezomib, the median PFS in the PAN-BTZ-Dex arm was 12.6 months (95% CI, 10.3-14.6) vs 9.2 months (95% CI, 8.1-11.5) in the Pbo-BTZ-Dex arm (HR, 0.69; 95% CI, 0.53-0.88; see supplemental Figure 1, available on the Blood Web site). Among patients with no prior history of IMiDs, the median PFS in the PAN-BTZ-Dex arm was 11.4 months (95% CI, 8.6-14.2) vs 12.0 months (95% CI, 9.0-13.1) in the Pbo-BTZ-Dex arm (HR, 0.78; 95% CI, 0.57-1.08; supplemental Figure 2).
PFS based on prior treatment. Kaplan-Meier analysis of PFS for each prior treatment subgroup. (A) Kaplan-Meier analysis of PFS among patients who received prior treatment with an IMiD. (B) Kaplan-Meier analysis of PFS among patients who received prior treatment with bortezomib and an IMiD. (C) PFS among patients who received ≥2 prior regimens including bortezomib and an IMiD.
PFS based on prior treatment. Kaplan-Meier analysis of PFS for each prior treatment subgroup. (A) Kaplan-Meier analysis of PFS among patients who received prior treatment with an IMiD. (B) Kaplan-Meier analysis of PFS among patients who received prior treatment with bortezomib and an IMiD. (C) PFS among patients who received ≥2 prior regimens including bortezomib and an IMiD.
Secondary end points of efficacy, including nCR/CR rate, time to response, duration of response, and time to progression, were also consistently improved among patients in the PAN-BTZ-Dex arm in each of the prior treatment groups (Figure 2; Table 2). The rate of high-quality responses (nCR/CR rate) was more than doubled among patients in the PAN-BTZ-Dex arm across all subgroups, with a notable increase among patients with ≥2 prior regimens including bortezomib and an IMiD: PAN-BTZ-Dex, 21.9% (95% CI, 13.1-33.1); Pbo-BTZ-Dex, 8.1% (95% CI, 3.0-16.8; Figure 2). Response duration was also increased in the PAN-BTZ-Dex group across all prior treatment groups (Table 2). Among patients who received prior bortezomib plus an IMiD, the response duration was 11.99 months (95% CI, 9.69-13.90) with PAN-BTZ-Dex and 8.31 months (95% CI, 6.14-12.32) with Pbo-BTZ-Dex. Similar to the results for PFS, the greatest difference in response duration was observed among patients with ≥2 prior regimens including bortezomib and an IMiD: PAN-BTZ-Dex, 11.99 months (95% CI, 9.69-13.37); Pbo-BTZ-Dex, 6.97 (95% CI, 4.86-13.40).
Response rate based on prior treatment. Response rate as demonstrated by ORR and nCR/CR is shown for each treatment arm, PAN-BTZ-Dex and Pbo-BTZ-Dex, and for each prior treatment subgroup analyzed: prior treatment with an IMiD, prior treatment with bortezomib and an IMiD, and patients who received ≥2 prior regimens including bortezomib and an IMiD. Statistical analyses were conducted for comparison of ORR between treatment arms.
Response rate based on prior treatment. Response rate as demonstrated by ORR and nCR/CR is shown for each treatment arm, PAN-BTZ-Dex and Pbo-BTZ-Dex, and for each prior treatment subgroup analyzed: prior treatment with an IMiD, prior treatment with bortezomib and an IMiD, and patients who received ≥2 prior regimens including bortezomib and an IMiD. Statistical analyses were conducted for comparison of ORR between treatment arms.
Secondary efficacy end points based on prior treatment
| . | Prior IMiD . | Prior BTZ + IMiD . | ≥2 prior regimens including BTZ and an IMiD . | |||
|---|---|---|---|---|---|---|
| PAN-BTZ-Dex (n = 245) . | Pbo-BTZ-Dex (n = 240) . | PAN-BTZ-Dex (n = 94) . | Pbo-BTZ-Dex (n = 99) . | PAN-BTZ-Dex (n = 73) . | Pbo-BTZ-Dex (n = 74) . | |
| Time to response, median (95% CI), mo | 1.45 (1.31-1.64) | 2.20 (1.54-6.34) | 1.58 (1.41-2.33) | NE (1.54-NE) | 1.54 (1.41-2.56) | NE (2.10-NE) |
| Duration of response, median (95% CI), mo | 13.14 (11.56-15.47) | 10.41 (7.95-11.53) | 11.99 (9.69-13.90) | 8.31 (6.14-12.32) | 11.99 (9.69-13.37) | 6.97 (4.86-13.40) |
| Time to progression/relapse/death, median (95% CI), mo | 12.68 (10.94-14.16) | 7.62 (6.31-8.31) | 12.25 (8.08-14.03) | 6.05 (4.70-7.56) | 12.68 (8.34-14.19) | 4.99 (3.75-6.80) |
| . | Prior IMiD . | Prior BTZ + IMiD . | ≥2 prior regimens including BTZ and an IMiD . | |||
|---|---|---|---|---|---|---|
| PAN-BTZ-Dex (n = 245) . | Pbo-BTZ-Dex (n = 240) . | PAN-BTZ-Dex (n = 94) . | Pbo-BTZ-Dex (n = 99) . | PAN-BTZ-Dex (n = 73) . | Pbo-BTZ-Dex (n = 74) . | |
| Time to response, median (95% CI), mo | 1.45 (1.31-1.64) | 2.20 (1.54-6.34) | 1.58 (1.41-2.33) | NE (1.54-NE) | 1.54 (1.41-2.56) | NE (2.10-NE) |
| Duration of response, median (95% CI), mo | 13.14 (11.56-15.47) | 10.41 (7.95-11.53) | 11.99 (9.69-13.90) | 8.31 (6.14-12.32) | 11.99 (9.69-13.37) | 6.97 (4.86-13.40) |
| Time to progression/relapse/death, median (95% CI), mo | 12.68 (10.94-14.16) | 7.62 (6.31-8.31) | 12.25 (8.08-14.03) | 6.05 (4.70-7.56) | 12.68 (8.34-14.19) | 4.99 (3.75-6.80) |
Safety
Mean duration of treatment was analyzed for all prior treatment groups: prior IMiD (PAN-BTZ-Dex, 6.67 months; Pbo-BTZ-Dex, 6.03 months), prior bortezomib plus IMiD (PAN-BTZ-Dex, 6.41 months; Pbo-BTZ-Dex, 5.13 months), and ≥2 prior regimens including bortezomib and an IMiD (PAN-BTZ-Dex, 6.42 months; Pbo-BTZ-Dex, 4.85 months). The median duration of treatment among each subgroup was as follows: prior IMiD (PAN-BTZ-Dex, 5.4 months vs Pbo-BTZ-Dex, 5.9 months); prior bortezomib plus IMiD (PAN-BTZ-Dex, 4.6 months vs Pbo-BTZ-Dex, 5.0 months); and ≥2 prior regimens including bortezomib and an IMiD (PAN-BTZ-Dex, 4.5 months vs Pbo-BTZ-Dex, 4.8 months).
A summary of dose changes/delays and median dose intensity for each drug across treatment arms and prior treatment groups is shown in supplemental Tables 1-4. The percentage of patients who had dose changes or delays of panobinostat/placebo, bortezomib, and dexamethasone were higher in the PAN-BTZ-Dex arm than in the Pbo-BTZ-Dex arm across all subgroups. Among patients who received ≥2 prior regimens including bortezomib and an IMiD, the percentage of patients who had ≥1 dose change were as follows: PAN-BTZ-Dex arm (PAN, 57%; BTZ, 61%; Dex, 32%) and Pbo-BTZ-Dex arm (Pbo, 30%; BTZ, 40%; Dex, 15%). Dose changes and delays were mostly due to AEs. Patients on the PAN-BTZ-Dex arm also received a lower median dose of all 3 drugs compared with those on the Pbo-BTZ-Dex arm across all prior treatment groups. The relative median dose intensity of each drug for patients who received ≥2 prior regimens including bortezomib and an IMiD were as follows: PAN-BTZ-Dex arm (PAN, 76.3%; BTZ, 72.5%; Dex, 83.3%) and Pbo-BTZ-Dex arm (Pbo, 91.6%; BTZ, 84.5%; Dex, 93.1%).
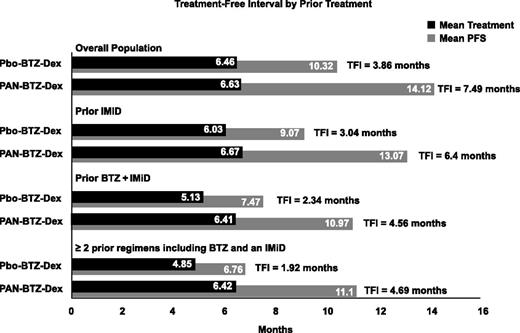
The TFI was determined for patients in each treatment arm for the overall population and for each subgroup. The TFI for each subgroup is shown in Figure 3. Overall, the TFI for the Pbo-BTZ-Dex arm was lower than that for the PAN-BTZ-Dex arm across all groups. Notably, in the subgroup of patients who received ≥2 prior regimens including bortezomib and an IMiD, patients in the Pbo-BTZ-Dex arm experienced a TFI of 1.92 months, whereas patients in the PAN-BTZ-Dex arm experienced a TFI of 4.69 months (Figure 3). This is substantiated by the results with the median treatment duration and median PFS between each of these subgroups outlined above. Notably, among patients who received ≥2 prior regimens including bortezomib and an IMiD, in the PAN-BTZ-Dex arm, median duration of treatment was 4.5 months and median PFS was 12.5 months, whereas in the Pbo-BTZ-Dex arm, median duration of treatment and median PFS were 4.8 and 4.7 months, respectively.
TFI by prior treatment. TFIs determined by subtracting the mean treatment duration from the mean PFS. TFIs are shown for each treatment arm for the overall treatment population and each prior treatment subgroup.
TFI by prior treatment. TFIs determined by subtracting the mean treatment duration from the mean PFS. TFIs are shown for each treatment arm for the overall treatment population and each prior treatment subgroup.
Overall, the safety profile of PAN-BTZ-Dex was similar among the prior treatment subgroups (Tables 3 and 4). The overall and grade 3/4 incidences of AEs and hematologic laboratory abnormalities that are known to be elevated in the PAN-BTZ-Dex arm vs the Pbo-BTZ-Dex arm (eg, diarrhea, fatigue/asthenia, thrombocytopenia, and neutropenia) were similarly elevated across all subgroups. The incidences of grade 3/4 diarrhea in the PAN-BTZ-Dex vs Pbo-BTZ-Dex arms across the 3 prior treatment groups were as follows: prior IMiD (26.1% vs 7.9%), prior bortezomib plus IMiD (30.4% vs 13.1%), and ≥2 prior regimens including bortezomib and an IMiD (33.3% vs 15.1%). Grade 3/4 thrombocytopenia across the 3 prior treatment groups was as follows for PAN-BTZ-Dex vs Pbo-BTZ-Dex: prior IMiD (61% vs 36%), prior bortezomib plus IMiD (68.5% vs 48.0%), and ≥2 prior regimens including bortezomib and an IMiD (68.1% vs 44.4%). A safety summary with relative risk for each of the prior treatment subgroups is provided in supplemental Tables 5 to 7.
Nonhematologic AEs observed in at least 20% of patients in any arm
| . | Prior IMiD . | Prior BTZ + IMiD . | ≥2 prior regimens including BTZ and an IMiD . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAN-BTZ-Dex (n = 241) . | Pbo-BTZ-Dex (n = 239) . | PAN-BTZ-Dex (n = 92) . | Pbo-BTZ-Dex (n = 99) . | PAN-BTZ-Dex (n = 72) . | Pbo-BTZ-Dex (n = 73) . | |||||||
| AE, n (%) . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . |
| Diarrhea | 167 (69) | 63 (26) | 97 (41) | 19 (8) | 67 (73) | 28 (30) | 46 (47) | 13 (13) | 55 (76) | 24 (33) | 34 (47) | 11 (15) |
| Fatigue/asthenia | 144 (60) | 61 (25) | 93 (39) | 28 (12) | 55 (60) | 23 (25) | 44 (44) | 12 (12) | 43 (60) | 19 (26) | 36 (49) | 10 (14) |
| Peripheral neuropathy | 149 (62) | 41 (17) | 160 (67) | 34 (14) | 51 (55) | 14 (15) | 52 (53) | 9 (9) | 42 (58) | 12 (17) | 39 (53) | 5 (7) |
| Nausea | 89 (37) | 14 (6) | 54 (23) | 2 (1) | 35 (38) | 8 (9) | 21 (21) | 1 (1) | 27 (38) | 8 (11) | 16 (22) | 1 (1) |
| Peripheral edema | 70 (29) | 4 (2) | 48 (20) | 0 | 18 (20) | 2 (2) | 18 (18) | 0 | 16 (22) | 2 (3) | 11 (15) | 0 |
| Vomiting | 65 (27) | 17 (7) | 32 (13) | 4 (2) | 24 (26) | 6 (7) | 10 (10) | 2 (2) | 18 (25) | 4 (6) | 7 (10) | 2 (3) |
| Hypokalemia | 65 (27) | 47 (20) | 30 (13) | 11 (5) | 24 (26) | 19 (21) | 17 (17) | 6 (6) | 18 (25) | 15 (21) | 12 (16) | 5 (7) |
| Decreased appetite | 62 (26) | 6 (3) | 30 (13) | 3 (1) | 21 (23) | 1 (1) | 13 (13) | 0 | 16 (22) | 1 (1) | 10 (14) | 0 |
| Upper respiratory tract infection | 60 (25) | 7 (3) | 40 (17) | 4 (2) | 30 (33) | 4 (4) | 17 (17) | 0 | 21 (29) | 4 (6) | 12 (16) | 0 |
| Pyrexia | 62 (26) | 3 (1) | 34 (14) | 5 (2) | 14 (15) | 0 | 13 (13) | 3 (3) | 10 (14) | 0 | 10 (14) | 3 (4) |
| Constipation | 59 (25) | 3 (1) | 73 (31) | 3 (1) | 25 (27) | 2 (2) | 32 (32) | 2 (2) | 19 (26) | 2 (3) | 20 (27) | 2 (3) |
| Cough | 54 (22) | 1 (0.4) | 46 (19) | 0 | 22 (24) | 0 | 20 (20) | 0 | 19 (26) | 0 | 15 (21) | 0 |
| Abdominal pain | 40 (17) | 5 (2) | 28 (12) | 3 (1) | 21 (23) | 2 (2) | 11 (11) | 2 (2) | 17 (24) | 1 (1) | 8 (11) | 2 (3) |
| . | Prior IMiD . | Prior BTZ + IMiD . | ≥2 prior regimens including BTZ and an IMiD . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAN-BTZ-Dex (n = 241) . | Pbo-BTZ-Dex (n = 239) . | PAN-BTZ-Dex (n = 92) . | Pbo-BTZ-Dex (n = 99) . | PAN-BTZ-Dex (n = 72) . | Pbo-BTZ-Dex (n = 73) . | |||||||
| AE, n (%) . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . |
| Diarrhea | 167 (69) | 63 (26) | 97 (41) | 19 (8) | 67 (73) | 28 (30) | 46 (47) | 13 (13) | 55 (76) | 24 (33) | 34 (47) | 11 (15) |
| Fatigue/asthenia | 144 (60) | 61 (25) | 93 (39) | 28 (12) | 55 (60) | 23 (25) | 44 (44) | 12 (12) | 43 (60) | 19 (26) | 36 (49) | 10 (14) |
| Peripheral neuropathy | 149 (62) | 41 (17) | 160 (67) | 34 (14) | 51 (55) | 14 (15) | 52 (53) | 9 (9) | 42 (58) | 12 (17) | 39 (53) | 5 (7) |
| Nausea | 89 (37) | 14 (6) | 54 (23) | 2 (1) | 35 (38) | 8 (9) | 21 (21) | 1 (1) | 27 (38) | 8 (11) | 16 (22) | 1 (1) |
| Peripheral edema | 70 (29) | 4 (2) | 48 (20) | 0 | 18 (20) | 2 (2) | 18 (18) | 0 | 16 (22) | 2 (3) | 11 (15) | 0 |
| Vomiting | 65 (27) | 17 (7) | 32 (13) | 4 (2) | 24 (26) | 6 (7) | 10 (10) | 2 (2) | 18 (25) | 4 (6) | 7 (10) | 2 (3) |
| Hypokalemia | 65 (27) | 47 (20) | 30 (13) | 11 (5) | 24 (26) | 19 (21) | 17 (17) | 6 (6) | 18 (25) | 15 (21) | 12 (16) | 5 (7) |
| Decreased appetite | 62 (26) | 6 (3) | 30 (13) | 3 (1) | 21 (23) | 1 (1) | 13 (13) | 0 | 16 (22) | 1 (1) | 10 (14) | 0 |
| Upper respiratory tract infection | 60 (25) | 7 (3) | 40 (17) | 4 (2) | 30 (33) | 4 (4) | 17 (17) | 0 | 21 (29) | 4 (6) | 12 (16) | 0 |
| Pyrexia | 62 (26) | 3 (1) | 34 (14) | 5 (2) | 14 (15) | 0 | 13 (13) | 3 (3) | 10 (14) | 0 | 10 (14) | 3 (4) |
| Constipation | 59 (25) | 3 (1) | 73 (31) | 3 (1) | 25 (27) | 2 (2) | 32 (32) | 2 (2) | 19 (26) | 2 (3) | 20 (27) | 2 (3) |
| Cough | 54 (22) | 1 (0.4) | 46 (19) | 0 | 22 (24) | 0 | 20 (20) | 0 | 19 (26) | 0 | 15 (21) | 0 |
| Abdominal pain | 40 (17) | 5 (2) | 28 (12) | 3 (1) | 21 (23) | 2 (2) | 11 (11) | 2 (2) | 17 (24) | 1 (1) | 8 (11) | 2 (3) |
Hematologic laboratory abnormalities
| AE, n (%) . | Prior IMiD . | Prior BTZ + IMiD . | ≥2 prior regimens including BTZ and an IMiD . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAN-BTZ-Dex (n = 241) . | Pbo-BTZ-Dex (n = 239) . | PAN-BTZ-Dex (n = 92) . | Pbo-BTZ-Dex (n = 99) . | PAN-BTZ-Dex (n = 72) . | Pbo-BTZ-Dex (n = 73) . | |||||||
| All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | |
| Thrombocytopenia | 234 (97) | 162 (67) | 199 (84) | 85 (36) | 89 (97) | 63 (69) | 88 (90) | 47 (48) | 70 (97) | 49 (68) | 65 (90) | 32 (44) |
| Leukopenia | 199 (83) | 57 (24) | 121 (51) | 24 (10) | 77 (84) | 18 (20) | 54 (55) | 12 (12) | 60 (83) | 15 (21) | 40 (55) | 8 (11) |
| Lymphopenia | 202 (84) | 130 (54) | 177 (74) | 97 (41) | 76 (83) | 46 (50) | 74 (75) | 46 (47) | 60 (83) | 35 (49) | 56 (77) | 36 (49) |
| Neutropenia | 193 (80) | 89 (37) | 86 (36) | 30 (13) | 74 (80) | 33 (36) | 45 (46) | 17 (17) | 60 (83) | 29 (40) | 33 (45) | 12 (16) |
| Anemia | 145 (60) | 41 (17) | 133 (56) | 51 (21) | 53 (58) | 17 (19) | 55 (56) | 19 (19) | 42 (58) | 15 (21) | 42 (58) | 15 (21) |
| AE, n (%) . | Prior IMiD . | Prior BTZ + IMiD . | ≥2 prior regimens including BTZ and an IMiD . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAN-BTZ-Dex (n = 241) . | Pbo-BTZ-Dex (n = 239) . | PAN-BTZ-Dex (n = 92) . | Pbo-BTZ-Dex (n = 99) . | PAN-BTZ-Dex (n = 72) . | Pbo-BTZ-Dex (n = 73) . | |||||||
| All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | All . | Grade 3/4 . | |
| Thrombocytopenia | 234 (97) | 162 (67) | 199 (84) | 85 (36) | 89 (97) | 63 (69) | 88 (90) | 47 (48) | 70 (97) | 49 (68) | 65 (90) | 32 (44) |
| Leukopenia | 199 (83) | 57 (24) | 121 (51) | 24 (10) | 77 (84) | 18 (20) | 54 (55) | 12 (12) | 60 (83) | 15 (21) | 40 (55) | 8 (11) |
| Lymphopenia | 202 (84) | 130 (54) | 177 (74) | 97 (41) | 76 (83) | 46 (50) | 74 (75) | 46 (47) | 60 (83) | 35 (49) | 56 (77) | 36 (49) |
| Neutropenia | 193 (80) | 89 (37) | 86 (36) | 30 (13) | 74 (80) | 33 (36) | 45 (46) | 17 (17) | 60 (83) | 29 (40) | 33 (45) | 12 (16) |
| Anemia | 145 (60) | 41 (17) | 133 (56) | 51 (21) | 53 (58) | 17 (19) | 55 (56) | 19 (19) | 42 (58) | 15 (21) | 42 (58) | 15 (21) |
The number of on-treatment deaths, defined as deaths occurring on treatment or up to 28 days after treatment, was higher in the PAN-BTZ-Dex arm (n = 17; 7.1%) than in the Pbo-BTZ-Dex arm (n = 10; 4.2%; Table 5) among patients who received prior IMiD. Among patients in this group, deaths due to progressive disease were similar (PAN-BTZ-Dex, n = 3; Pbo-BTZ-Dex, n = 4), but there were a higher number of deaths due to other causes (primarily AEs) in the PAN-BTZ-Dex arm (PAN-BTZ-Dex, n = 14; Pbo-BTZ-Dex, n = 6). The number of on-treatment deaths was similar between treatment arms in the prior bortezomib plus IMiD group (PAN-BTZ-Dex, n = 6 [6.5%]; Pbo-BTZ-Dex, n = 5 [5.1%]) and in the group with ≥2 prior regimens including bortezomib and an IMiD (PAN-BTZ-Dex, n = 5 [6.9%]; Pbo-BTZ-Dex, n = 5 [6.8%]). Within these 2 groups, no deaths due to progressive disease were reported for patients in the PAN-BTZ-Dex arm, whereas 2 deaths were reported in the Pbo-BTZ-Dex arm. The number of deaths due to other causes was slightly higher in the PAN-BTZ-Dex arm than in the Pbo-BTZ-Dex arm in both subgroups: prior bortezomib plus IMiD (PAN-BTZ-Dex, n = 6 [6.5%]; Pbo-BTZ-Dex, n = 3 [3.0%]); ≥2 prior regimens including bortezomib and an IMiD (PAN-BTZ-Dex, n = 5 [6.9%]; Pbo-BTZ-Dex, n = 3 [4.1%]).
On-treatment deaths
| Deaths, n (%) . | Prior IMiD . | Prior BTZ + IMiD . | ≥2 prior regimens including BTZ and an IMiD . | |||
|---|---|---|---|---|---|---|
| PAN-BTZ-Dex (n = 241) . | Pbo-BTZ-Dex (n = 239) . | PAN-BTZ-Dex (n = 92) . | Pbo-BTZ-Dex (n = 99) . | PAN-BTZ-Dex (n = 72) . | Pbo-BTZ-Dex (n = 73) . | |
| Total | 17 (7.1) | 10 (4.2) | 6 (6.5) | 5 (5.1) | 5 (6.9) | 5 (6.8) |
| Due to progressive disease | 3 (1.2) | 4 (1.7) | 0 | 2 (2.0) | 0 | 2 (2.7) |
| Due to other causes | 14 (5.8) | 6 (2.5) | 6 (6.5) | 3 (3.0) | 5 (6.9) | 3 (4.1) |
| Cardiac disorders | ||||||
| Myocardial infarction | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Myocardial ischemia | 1 (0.4) | 0 | 1 (1.1) | 0 | 1 (1.4) | 0 |
| Cardiorespiratory arrest | 0 | 1 (0.4) | 0 | 1 (1.0) | 0 | 1 (1.4) |
| Cardiopulmonary failure | 0 | 1 (0.4) | 0 | 0 | 0 | 0 |
| Gastrointestinal disorders | ||||||
| Gastrointestinal hemorrhage | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Intestinal ischemia | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| General disorders and administration-site conditions | ||||||
| Death | 1 (0.4) | 0 | 1 (1.1) | 0 | 1 (1.4) | 0 |
| Infections and infestations | ||||||
| Lung infection | 1 (0.4) | 0 | 1 (1.1) | 0 | 1 (1.4) | 0 |
| Pneumonia | 1 (0.4) | 1 (0.4) | 0 | 0 | 0 | 0 |
| Septic shock | 1 (0.4) | 0 | 1 (1.1) | 0 | 0 | 0 |
| Necrotizing fasciitis | 0 | 1 (0.4) | 0 | 1 (1.0) | 0 | 1 (1.4) |
| Neutropenic sepsis | 0 | 1 (0.4) | 0 | 1 (1.0) | 0 | 1 (1.4) |
| Nervous system disorders | ||||||
| Cerebral hemorrhage | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Cerebrovascular accident | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Renal and urinary disorders | ||||||
| Acute renal failure | 2 (0.8) | 0 | 1 (1.1) | 0 | 1 (1.4) | 0 |
| Respiratory, thoracic, and mediastinal disorders | ||||||
| Pulmonary hemorrhage | 1 (0.4) | 0 | 1 (1.1) | 0 | 1 (1.4) | 0 |
| Respiratory failure | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Pulmonary embolism | 0 | 1 (0.4) | 0 | 0 | 0 | 0 |
| Deaths, n (%) . | Prior IMiD . | Prior BTZ + IMiD . | ≥2 prior regimens including BTZ and an IMiD . | |||
|---|---|---|---|---|---|---|
| PAN-BTZ-Dex (n = 241) . | Pbo-BTZ-Dex (n = 239) . | PAN-BTZ-Dex (n = 92) . | Pbo-BTZ-Dex (n = 99) . | PAN-BTZ-Dex (n = 72) . | Pbo-BTZ-Dex (n = 73) . | |
| Total | 17 (7.1) | 10 (4.2) | 6 (6.5) | 5 (5.1) | 5 (6.9) | 5 (6.8) |
| Due to progressive disease | 3 (1.2) | 4 (1.7) | 0 | 2 (2.0) | 0 | 2 (2.7) |
| Due to other causes | 14 (5.8) | 6 (2.5) | 6 (6.5) | 3 (3.0) | 5 (6.9) | 3 (4.1) |
| Cardiac disorders | ||||||
| Myocardial infarction | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Myocardial ischemia | 1 (0.4) | 0 | 1 (1.1) | 0 | 1 (1.4) | 0 |
| Cardiorespiratory arrest | 0 | 1 (0.4) | 0 | 1 (1.0) | 0 | 1 (1.4) |
| Cardiopulmonary failure | 0 | 1 (0.4) | 0 | 0 | 0 | 0 |
| Gastrointestinal disorders | ||||||
| Gastrointestinal hemorrhage | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Intestinal ischemia | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| General disorders and administration-site conditions | ||||||
| Death | 1 (0.4) | 0 | 1 (1.1) | 0 | 1 (1.4) | 0 |
| Infections and infestations | ||||||
| Lung infection | 1 (0.4) | 0 | 1 (1.1) | 0 | 1 (1.4) | 0 |
| Pneumonia | 1 (0.4) | 1 (0.4) | 0 | 0 | 0 | 0 |
| Septic shock | 1 (0.4) | 0 | 1 (1.1) | 0 | 0 | 0 |
| Necrotizing fasciitis | 0 | 1 (0.4) | 0 | 1 (1.0) | 0 | 1 (1.4) |
| Neutropenic sepsis | 0 | 1 (0.4) | 0 | 1 (1.0) | 0 | 1 (1.4) |
| Nervous system disorders | ||||||
| Cerebral hemorrhage | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Cerebrovascular accident | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Renal and urinary disorders | ||||||
| Acute renal failure | 2 (0.8) | 0 | 1 (1.1) | 0 | 1 (1.4) | 0 |
| Respiratory, thoracic, and mediastinal disorders | ||||||
| Pulmonary hemorrhage | 1 (0.4) | 0 | 1 (1.1) | 0 | 1 (1.4) | 0 |
| Respiratory failure | 1 (0.4) | 0 | 0 | 0 | 0 | 0 |
| Pulmonary embolism | 0 | 1 (0.4) | 0 | 0 | 0 | 0 |
Discussion
These analyses demonstrate that the addition of the deacetylase inhibitor panobinostat to bortezomib and dexamethasone improves efficacy outcomes in patients with MM following treatment with ≥2 prior regimens including IMiDs and bortezomib, a population with a poorer prognosis and an urgent unmet need. In this population, there was an increase in median PFS of 7.8 months for the PAN-BTZ-Dex arm vs the Pbo-BTZ-Dex arm. These data highlight two key observations. First, the results suggest that this heavily pretreated population benefits from the addition of panobinostat to bortezomib and dexamethasone treatment. The median PFS in patients on the PAN-BTZ-Dex arm who received at least 2 prior regimens including bortezomib and an IMiD was comparable to that in the overall population (12.5 months vs 12.0 months, respectively), supporting the role of deacetylase inhibitors in relapsed or relapsed and refractory MM. The second observation is that patients who received at least 2 prior regimens including bortezomib and an IMiD demonstrated poorer outcomes with bortezomib plus dexamethasone alone. Notably, in this population, median PFS in the Pbo-BTZ-Dex arm was only 4.7 months, confirming the poor prognosis in these patients and supporting earlier observations that panobinostat recaptures responses in more heavily pretreated patients.10,11 The data from the current analyses support the recent approvals by the US Food and Drug Administration and European Commission for use of panobinostat in combination with bortezomib and dexamethasone in patients who have received ≥2 prior regimens including bortezomib and an IMiD, support the results of the primary analysis of the PANORAMA 1 study, and provide insights for use of PAN-BTZ-Dex in the clinic.6
Panobinostat represents a novel addition to the MM treatment armamentarium by introducing an agent with a novel mechanism of action.4-6,11 Novel agents are needed to address the ongoing unmet need in patients who progress on bortezomib and IMiDs as a strategy to overcome therapeutic resistance. Recent developments in the treatment of patients with resistance to bortezomib and IMiDs have been limited to newer proteasome inhibitors (eg, carfilzomib) and IMiDs (eg, pomalidomide). Both carfilzomib and pomalidomide have shown clear efficacy in patients who have progressed following treatment with bortezomib and thalidomide or lenalidomide, both as a single agent and in combination.12-15 However, as panobinostat acts on distinct epigenetic and protein metabolism pathways, it is uniquely suited to provide benefit in patients previously treated with proteasome inhibitors and/or IMiDs.4,5
Although regimens currently used in the treatment of relapsed and/or refractory MM are associated with a relatively long PFS, particularly in nonrefractory populations, they are administered until progression in clinical trials.12-14 Although the primary goal for patients with previously treated MM is to prolong the duration of remission, the TFI in patients with MM has been shown to have a positive impact on patients by allowing them to recover from treatment-associated toxicities.16 PAN-BTZ-Dex demonstrated a TFI of 4.7 months in heavily pretreated patients who received ≥2 prior regimens including bortezomib and an IMiD, whereas the TFI for patients in the Pbo-BTZ-Dex arm was 1.9 months.
In addition to the PFS benefit among patients on the PAN-BTZ-Dex arm that was maintained across all subgroups, the rate of deep responses (nCR/CR) was also increased. This is noteworthy among the most heavily pretreated population of patients who received ≥2 prior lines including bortezomib and an IMiD. In this population, the nCR/CR rate was 22% for the PAN-BTZ-Dex arm vs 8% for the Pbo-BTZ-Dex arm. Achievement of deep responses is of interest, particularly in heavily pretreated relapsed or relapsed and refractory patient populations, because treatment options are limited and deeper responses are associated with improved clinical outcomes, including PFS and OS.17,18
Although the results of the PANORAMA 1 trial demonstrated a clear benefit in efficacy for the PAN-BTZ-Dex arm, there was also an increase in AEs and hematologic laboratory abnormalities and on-treatment deaths.6 Common AEs and laboratory abnormalities in the PAN-BTZ-Dex arm among all prior treatment subgroups analyzed were similar to those of the overall PANORAMA 1 population. Diarrhea was the most common nonhematologic AE, and thrombocytopenia was the most common hematologic laboratory abnormality. Rates of these AEs were somewhat more pronounced in patients in the Pbo-BTZ-Dex arm of the more heavily pretreated subgroups. Overall, these results suggest that, in general, the safety profile remains consistent regardless of prior treatment and adverse events are manageable with dose interruptions or reductions and supportive measures for key toxicities (eg, loperamide for diarrhea and platelet transfusion for thrombocytopenia).19,20 In the overall PANORAMA 1 study population, there were slightly more on-treatment deaths in the PAN-BTZ-Dex arm vs the Pbo-BTZ-Dex arm (n = 30 [8%] vs n = 18 [5%], respectively). The proportions of on-treatment deaths were similar among the subgroup of patients who received ≥2 prior regimens including bortezomib and an IMiD (PAN-BTZ-Dex, n = 5 [6.9%]; Pbo-BTZ-Dex, n = 5 [6.8%]). These data also suggest that the risk associated with PAN-BTZ-Dex is reduced among the patient population with a poorer prognosis.
Overall, the results of this analysis of patients in the PANORAMA 1 study by prior treatment demonstrate that patients with relapsed or relapsed and refractory MM on the PAN-BTZ-Dex arm who received prior bortezomib plus IMiD derive a greater benefit in PFS and a better benefit:risk profile. As treatment options for relapsed MM increase with the development of novel therapies and treatment combinations, it is critical to identify patient populations that derive the greatest benefit to aid physicians in treatment decisions. These results provide guidance as to which patients may benefit more from the PAN-BTZ-Dex combination. Investigation of other novel panobinostat combinations continues, including combinations with carfilzomib, ixazomib, and lenalidomide, in order to identify new treatment alternatives for patients with limited options and provide additional insight on the safety profile of this novel agent.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ashok Panneerselvam, Novartis Pharmaceuticals Corporation, for statistical support. The authors also thank William Fazzone, PhD, Articulate Science, for editorial writing support, funded by Novartis Pharmaceuticals Corporation.
The PANORAMA 1 study was funded by Novartis Pharmaceuticals Corporation.
Authorship
Contribution: P.G.R., S.L., J.H.L., and J.F.S.-M. contributed to study conception and design; V.T.M.H., S.-S.Y., M.B., M.A.D., A.E., W.W.J., A.G., R.L.S., J.H., P.M., J.H.L., H.E., and J.F.S.-M. contributed to provision of study materials and patients; S.-S.Y., M.B., M.A.D., A.E., W.W.J., A.G., T.N.N., N.S., J.H., J.H.L., and H.E. collected data; B.-R.B. performed statistical analyses; P.G.R., P.M., S.L., J.H.L., H.E., M.S., C.C., B.-R.B., F.B., and J.F.S.-M. analyzed and interpreted data; and all authors drafted and approved the manuscript.
Conflict-of-interest disclosure: V.T.M.H., S.-S.Y., A.E., T.N.N., and J.H.L. declare no competing financial interests. P.G.R. has served on advisory committees for Novartis, Takeda, Johnson & Johnson, and Celgene and has received research funding from Celgene and Takeda. M.B. has received honoraria from Novartis and Takeda; participated in speakers' bureau for Janssen-Cilag, Celgene, Bristol-Myers Squibb, and Amgen; and has served on advisory committees or boards of directors for Amgen, Novartis, Takeda, and Bristol-Myers Squibb. M.A.D. has received honoraria from Celgene, Janssen, Novartis, Onyx, and Amgen. W.W.J. has received research funding from Novartis and Amgen and has served on advisory committees for Celgene, Amgen, Roche, Janssen, and Onconova. A.G. has served as a consultant and on advisory committees and received honoraria from Novartis. R.L.S. has served on advisory committees or board of directors for Millennium. N.S. has received research funding from Janssen-Cilag, Novartis, Roche, and Pfizer. J.H. has served on advisory committees or board of directors for Novartis. P.M. has received honoraria from Novartis, Janssen, Amgen, Celgene, and Takeda. S.L. has served in a consultant role for Millennium, Celgene, Novartis, Bristol-Myers Squibb, Onyx, and Janssen. H.E. has served in a consultant role, received research funding and honoraria from Celgene, Janssen, Amgen/Onyx and Novartis; and has served on a speakers’ bureau for Celgene and Janssen. J.F.S.-M. has served on advisory committees or boards of directors for Millennium, Celgene, Novartis, Onyx, Janssen, Bristol-Myers Squibb, and Merck Sharpe & Dohme. M.S., C.C., B.-R.B., and F.B. are employees of Novartis.
Correspondence: Paul G. Richardson, Dana-Farber Cancer Institute, Harvard University, 450 Brookline Ave, Mailstop: Dana 1B02, Boston, MA 02215; e-mail: paul_richardson@dfci.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal