Key Points
High-density lipoprotein and its major apolipoprotein ApoA-I prevent von Willebrand factor self-association.
Targeting von Willebrand factor self-association could be a new approach to treating thrombotic disorders.
Abstract
The ability of von Willebrand factor (VWF) to initiate platelet adhesion depends on the number of monomers in individual VWF multimers and on the self-association of individual VWF multimers into larger structures. VWF self-association is accelerated by shear stress. We observed that VWF self-association occurs during adsorption of VWF onto surfaces, assembly of secreted VWF into hyperadhesive VWF strings on the endothelial surface, and incorporation of fluid-phase VWF into VWF fibers. VWF adsorption under static conditions increased with increased VWF purity and was prevented by a component of plasma. We identified that component as high-density lipoprotein (HDL) and its major apolipoprotein ApoA-I. HDL and ApoA-I also prevented VWF on the endothelium from self-associating into longer strands and inhibited the attachment of fluid-phase VWF onto vessel wall strands. Platelet adhesion to VWF fibers was reduced in proportion to the reduction in self-associated VWF. In a mouse model of thrombotic microangiopathy, HDL also largely prevented the thrombocytopenia induced by injection of high doses of human VWF. Finally, a potential role for ApoA-I in microvascular occlusion associated with thrombotic thrombocytopenic purpura and sepsis was revealed by the inverse relationship between the concentration of ApoA-I and that of hyperadhesive VWF. These results suggest that interference with VWF self-association would be a new approach to treating thrombotic disorders.
Introduction
A number of acute systemic diseases, including thrombotic thrombocytopenic purpura (TTP),1,2 sepsis,3 malaria,4 and sickle cell disease,5 are characterized by systemic endothelial activation and microvascular occlusion. In recent studies, von Willebrand factor (VWF) secreted from the endothelium as a result of systemic endothelial activation has been shown to contribute to microvascular dysfunction and occlusion. A portion of the newly secreted VWF remains attached to the endothelial surface, from which it is normally removed through cleavage by the plasma metalloprotease ADAMTS13.6 However, when ADAMTS13 is inhibited, as during episodes of TTP, or when VWF removal is delayed, as in conditions of intense inflammation such as sepsis and malaria,7 the secreted VWF self-associates into long, thick strands that bind platelets to form occlusive thrombi in the small blood vessels.6
VWF synthesis is a complex process. VWF homodimers first form in the endoplasmic reticulum through disulfide bonds involving cysteine residues near the VWF carboxyl terminus. In the Golgi apparatus, these dimers are linked together into chains through disulfide bonds at the amino termini, producing a ladder of multimeric molecules, each differing from the next largest by the molecular mass of a VWF dimer. The largest multimers found in blood can be enormous, reaching a molecular mass of more than 20 million Da. These gargantuan molecules can form even larger structures by associating with other VWF multimers. VWF self-association has been described on glass or collagen surfaces,8 on the endothelial surface to form strands,6,9 and on platelet surfaces.10 VWF self-association is promoted by fluid shear stress.9,11 We recently showed, in an in vitro system of endothelialized microvessels, that VWF secreted from the vessel wall is able to span the lumen of vessels with diameters up to 300 µm at sites of turns and bifurcations.9 There is evidence that after the VWF has self-associated, disulfide bonds between adjacent multimers can stabilize the association.12
The ability of VWF to self-associate is likely necessary for certain VWF functions. For example, self-associated VWF supports platelet adhesion much better than do individual VWF multimers, evidenced by the fact that platelets perfused over a single-layer VWF surface roll without adhering firmly,13 whereas they attach firmly without rolling to self-associated endothelial VWF strands.6 Self-association also allows VWF to form strands long enough to reach above the endothelial boundary layer under flow to bind platelets, which may explain the findings of a study showing that platelets bound only a subset of endothelial VWF strings identified with a VWF antibody.14
On the basis of our observation that highly purified VWF was lost from solution by first binding to the wall of the container and then by self-associating, we identified high-density lipoprotein (HDL) as the plasma component that prevents VWF self-association. We provide evidence that this is a novel and heretofore unknown antithrombotic property of this cardioprotective lipoprotein.
Methods
Patients and blood donors
Collection of blood samples from healthy donors was approved by the Western Institutional Review Board. Collection of blood samples from patients with sepsis and TTP was approved by the Institutional Review Board of the University of Washington. Twelve patients with sepsis were examined. These patients were hospitalized in the intensive care unit at Harborview Medical Center and had either isolated sepsis or sepsis with acute lung injury (supplemental Table 1, available on the Blood Web site). Sepsis was defined clinically by the presence of systemic inflammatory response syndrome and suspicion of or evidence of infection. Blood was also collected from 5 patients presumptively diagnosed with TTP on the basis of the clinical presentation of thrombocytopenia and microangiopathic hemolytic anemia (supplemental Table 2). One of these patients was later confirmed to have lupus-associated thrombotic microangiopathy. The blood was collected before initiation of plasmapheresis.
Mice
All of the procedures that used mice were approved by the Animal Care and Use Committee of Bloodworks Research Institute. Fourteen-week-old ADAMTS13-deficient (Adamts13−/−) mice were used. The mice had been backcrossed for more than 10 generations onto a C57BL/6 background.
Thrombotic microangiopathy in Adamts13 knockout mice
Experiments were performed as described previously.15 Briefly, we injected mice through their tail veins with VWF (5 mg/kg), VWF (5 mg/kg) plus HDL (2 mg), or saline and drew blood into 3.8% citrate from jugular veins before injection and 3 and 6 hours after injection. Both purified VWF and HDL (Sigma-Aldrich) were from human plasma. Platelets in whole blood were labeled with fluorescein isothiocyanate–conjugated rat anti-mouse CD41 in a Trucount tube (BD Bioscience) and quantified by flow cytometry. VWF in plasma was quantified by sandwich enzyme-linked immunosorbent assay.
Statistical Methods
Welch’s t test (assuming unequal variances between groups) was used to compare means of VWF string units on the endothelial surface between control (no HDL) and each of the conditions of HDL treatment (during stimulation, during perfusion, or both), with Bonferroni’s correction applied to P values to maintain an overall false-positive error rate across all 3 tests. Welch’s t test was also used to compare VWF levels in mouse plasma and normalized platelet counts (percent of baseline per mouse) in whole blood between mice injected with VWF and those injected with VWF plus HDL at 3 and 6 hours. Dunnett’s test was used to compare apolipoprotein A-I (ApoA-I), ADAMTS13 activity, and total active VWF levels in patients with sepsis and TTP with those in normal controls. Analyses were conducted by using Microsoft Excel and R software (http://www.R-project.org).
Results
HDL and ApoA-I prevent VWF surface adsorption and self-association
Adsorptive loss of purified VWF by self-association under static conditions.
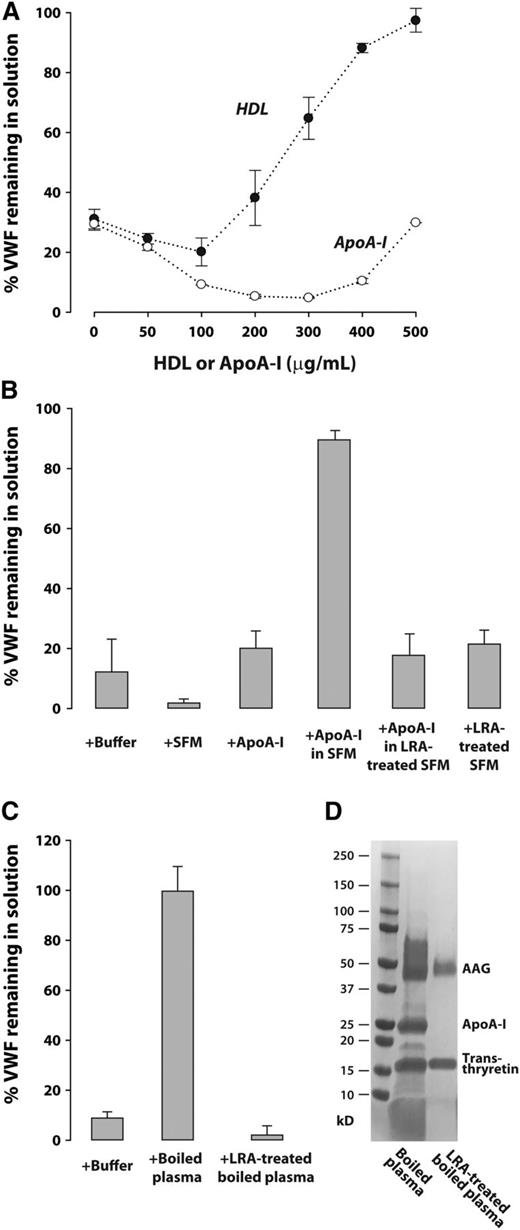
We noticed that highly purified VWF, whether the plasma form (supplemental Figure 1) or recombinant biotin-tagged form (Figure 1A, lane 2), rapidly decreased in concentration when placed in polyethylene tubes, a time-dependent phenomenon that did not occur with partially purified VWF. We reasoned that the protein was lost by adsorption to the tube surface, a phenomenon that has been studied by Magnani et al,16 who proposed that adsorption proceeds in 2 steps, the protein interacting first with the surface and subsequently with pre-adsorbed protein. The two types of adsorption can be distinguished by sequential elution with different detergents, as the authors described. By using a similar strategy, eluting first with sodium dodecyl sulfate and then with 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, we found that the majority (∼90%) of VWF was loosely bound, having been eluted with 2% SDS, and ∼10% could be eluted only with 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate containing urea and dithiothreitol (Figure 1A, lane 2), suggesting that this fraction of VWF was bound directly to plastic. The results are consistent with initial adsorption of VWF to plastic followed by self-association.
HDL/ApoA-I prevents VWF self-association under both static and shear conditions. (A) Boiled plasma and ApoA-I prevent VWF surface adsorption and self-association under static conditions. Purified recombinant Bio-VWF (8 μg/mL) in 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) and 2 mM CaCl2 was incubated with buffer or different proteins at 22°C for 4 hours in 1.5-mL microfuge tubes. At the end of incubations, unbound proteins remaining in solution were removed. Proteins adsorbed to the tube surfaces were sequentially eluted first with buffer containing sodium dodecyl sulfate (SDS) (2% SDS, 2 mM CaCl2, 10 mM HEPES, pH 7.4), and then with 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) elution buffer (4% CHAPS, 8 M urea, 5 mM dithiothreitol, 40 mM tris(hydroxymethyl)aminomethane [Tris], pH 9.5). Bio-VWF remaining in the fluid phase and that bound to the surface were both analyzed by western blot after electrophoresis on reducing polyacrylamide gels. (B) VWF self-association is enhanced by shear stress and prevented by HDL. Purified VWF from cryoprecipitate (7 μg/mL in 10 mM HEPES, 25 mM NaCl, 10 mM EDTA, pH 7.5), Bio-VWF in serum-free medium desalted into the buffer above at 10 μg/mL, and 50% citrated plasma in 10 mM EDTA were sheared by vortexing at 2400 rpm in a rotary mixer for 90 minutes at 37°C. VWF remaining in solution was quantified by enzyme-linked immunosorbent assay (ELISA) and expressed as a percentage of the VWF in parallel samples not exposed to shear stress (n = 3). The VWF multimer distribution was analyzed by SDS-agarose gel electrophoresis and western blotting as described in supplemental Data. (C) HDL/ApoA-I dose-dependently inhibits VWF self-association under shear stress. Samples of 50% citrated plasma in 10 mM EDTA were sheared as above in increasing concentrations of HDL and ApoA-I or in single concentrations of alpha-1-acid glycoprotein (AAG) and transthyretin (each at 500 μg/mL). VWF remaining in solution was determined by ELISA. (D) Correlation of VWF remaining in solution after exposure to shear stress with the amount of VWF–ApoA-I complex formation measured by sandwich ELISA is described in supplemental Data. BSA, bovine serum albumin.
HDL/ApoA-I prevents VWF self-association under both static and shear conditions. (A) Boiled plasma and ApoA-I prevent VWF surface adsorption and self-association under static conditions. Purified recombinant Bio-VWF (8 μg/mL) in 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) and 2 mM CaCl2 was incubated with buffer or different proteins at 22°C for 4 hours in 1.5-mL microfuge tubes. At the end of incubations, unbound proteins remaining in solution were removed. Proteins adsorbed to the tube surfaces were sequentially eluted first with buffer containing sodium dodecyl sulfate (SDS) (2% SDS, 2 mM CaCl2, 10 mM HEPES, pH 7.4), and then with 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) elution buffer (4% CHAPS, 8 M urea, 5 mM dithiothreitol, 40 mM tris(hydroxymethyl)aminomethane [Tris], pH 9.5). Bio-VWF remaining in the fluid phase and that bound to the surface were both analyzed by western blot after electrophoresis on reducing polyacrylamide gels. (B) VWF self-association is enhanced by shear stress and prevented by HDL. Purified VWF from cryoprecipitate (7 μg/mL in 10 mM HEPES, 25 mM NaCl, 10 mM EDTA, pH 7.5), Bio-VWF in serum-free medium desalted into the buffer above at 10 μg/mL, and 50% citrated plasma in 10 mM EDTA were sheared by vortexing at 2400 rpm in a rotary mixer for 90 minutes at 37°C. VWF remaining in solution was quantified by enzyme-linked immunosorbent assay (ELISA) and expressed as a percentage of the VWF in parallel samples not exposed to shear stress (n = 3). The VWF multimer distribution was analyzed by SDS-agarose gel electrophoresis and western blotting as described in supplemental Data. (C) HDL/ApoA-I dose-dependently inhibits VWF self-association under shear stress. Samples of 50% citrated plasma in 10 mM EDTA were sheared as above in increasing concentrations of HDL and ApoA-I or in single concentrations of alpha-1-acid glycoprotein (AAG) and transthyretin (each at 500 μg/mL). VWF remaining in solution was determined by ELISA. (D) Correlation of VWF remaining in solution after exposure to shear stress with the amount of VWF–ApoA-I complex formation measured by sandwich ELISA is described in supplemental Data. BSA, bovine serum albumin.
HDL and ApoA-I prevent VWF self-association.
The fact that only highly purified VWF was lost by adsorption suggested that other molecules in plasma prevented this phenomenon. We used a variety of techniques to determine the nature of the VWF stabilizing factor, including boiling the plasma for 10 minutes, which denatures and precipitates 98% of plasma proteins. The supernatant of boiled plasma still protected purified VWF from adsorptive loss (Figure 1A, lane 3; supplemental Figure 1) and contained only 3 abundant proteins (supplemental Figure 2): α1 acid glycoprotein (∼50 kDa; 0.73 mg/mL in plasma), ApoA-I (∼28 kDa; 1 mg/mL in plasma), and transthyretin (16 kDa; 0.22 mg/mL in plasma) (supplemental Table 3). Of the 3 proteins, ApoA-I was by far the most effective at preventing VWF adsorption and self-association (Figure 1A, lane 4 vs lanes 6 and 7). HDL particles, which contain the majority of the ApoA-I in circulation, also stabilized VWF (Figure 1A, lane 5). Lipid-free ApoA-I and native HDL particles inhibited VWF self-association to a similar extent, suggesting that ApoA-I is responsible for the effect. ApoA-I dose-dependently stabilized VWF and reproduced all of the VWF-stabilizing properties of boiled plasma (supplemental Figure 3).
Although it was able to prevent VWF surface adsorption, HDL was unable to elute surface-bound VWF (supplemental Figure 4). Adsorption of VWF was lowest when the surface was pre-coated with HDL and HDL was also present in the fluid phase (supplemental Figure 4). Thus, HDL prevents the interaction of fluid-phase VWF both directly with the surface and with surface-bound VWF.
The VWF A1A2A3 fragment binds immobilized VWF.
The A1A2A3 region of VWF is vital to VWF function, containing platelet and collagen binding sites and the ADAMTS13 cleavage site. We examined whether this region might be involved in VWF self-association. A recombinant Bio-A1A2A3 fragment bound surface-immobilized VWF (supplemental Figure 5), indicating that surface-immobilized VWF exposes sites that bind this region. Both HDL and ApoA-I prevented the binding of the fragment to immobilized VWF (supplemental Figure 5).
Shear stress enhances adsorptive loss of plasma VWF.
On the basis of our previous data showing that shear stress enhances VWF strand formation on the endothelial surface,9 we evaluated whether shearing plasma would overcome the protective effect of plasma HDL on VWF adsorption. For technical reasons, we chose to apply shear stress to the samples by vortexing them in the presence of EDTA (to inhibit ADAMTS13). Vortexing had little effect on VWF adsorptive loss until an rpm threshold between 1800 and 2400 was reached. Plasma sheared at 2400 rpm for 90 minutes lost approximately 80% of its VWF to adsorption (supplemental Figure 6A). In contrast, purified VWF and Bio-VWF in cell culture medium were rapidly and completely depleted from the fluid phase at the same shear stress (Figure 1B). Thus, VWF self-association induced by shear stress can occur in the presence of all plasma proteins, although the process is delayed compared with purified VWF, suggesting that plasma contains a limiting amount of VWF stabilizing agent. Addition of 0.5 mg/mL HDL to plasma (∼25% of the normal plasma HDL concentration) stabilized the entire range of VWF multimers in solution (Figure 1B). Both HDL and lipid-free ApoA-I dose-dependently stabilized VWF in plasma under shear (Figure 1C), accompanied by the formation of a soluble VWF–ApoA-I complex (Figure 1D). Although HDL was more efficient than ApoA-I in stabilizing VWF in this setting, the amount of VWF–ApoA-I complex formed correlated with the extent of VWF stabilization irrespective of whether HDL or ApoA-I was used (Figure 1D).
To evaluate the association of HDL with VWF, we added Bio-VWF to normal plasma and vortexed the plasma for 90 minutes at various speeds. The Bio-VWF remaining in solution was captured on streptavidin beads, and the associated ApoA-I was quantified by western blotting. The amount of ApoA-I in complex with Bio-VWF was significantly higher in the sample that was exposed to high shear (supplemental Figure 6B). These results show that HDL prevents VWF self-association by masking self-association sites exposed by shear stress.
ApoA-I requires lipid to stabilize VWF under shear
We next studied the influence of lipid or lipoproteins in plasma on VWF self-association by depleting plasma of these components with the lipid removal agent LRA, a synthetic crystal of calcium silicate hydrate. This agent also removed 95% of the VWF and 90% of the ADAMTS13 from plasma (data not shown). We added purified VWF to the delipidated plasma and evaluated VWF stabilization by HDL and ApoA-I under shear stress. Under these conditions, HDL, but not ApoA-I, dose-dependently stabilized VWF, suggesting that ApoA-I must first associate with lipid to perform this task (Figure 2A). Curiously, addition of ApoA-I to the delipidated plasma at lower concentrations appeared to increase VWF adsorption. Consistent with the requirement for lipid, ApoA-I in serum-free cell culture medium was able to stabilize VWF in delipidated plasma (Figure 2B) but not if the serum-free cell culture medium had been delipidated before combining with ApoA-I (Figure 2B). Boiled plasma could also stabilize VWF when added to delipidated plasma (Figure 2C), a property that was also lost when the boiled plasma was treated with LRA, which removed ApoA-I (Figure 2C-D).
ApoA-I requires lipid to prevent VWF self-association under shear stress. Purified VWF (7 μg/mL) was added to LRA-treated plasma with 10 mM EDTA and sheared at 2400 rpm for 90 minutes at 37°C. The VWF remaining in solution after exposure to shear stress was expressed as a percentage of VWF solution in parallel samples not exposed to shear. (A) Purified VWF in LRA-treated plasma was sheared in the presence of increasing concentrations of HDL or ApoA-I. (B) Purified VWF in LRA-treated plasma was sheared in the presence of buffer (10 mM HEPES, 25 mM NaCl, 10 mM EDTA, pH 7.5), desalted serum-free medium (SFM), LRA-treated SFM, purified ApoA-I (500 μg/mL), and combinations of these reagents. (C) Purified VWF in LRA-treated plasma was sheared in the presence of boiled plasma proteins (500 μg/mL) before or after treatment with LRA. (D) SDS-gradient polyacrylamide gel electrophoresis (4% to 15%) and Gelcode blue stain (Thermo Scientific) of boiled plasma (15 μg protein) and boiled plasma after treatment with LRA (8 μg protein).
ApoA-I requires lipid to prevent VWF self-association under shear stress. Purified VWF (7 μg/mL) was added to LRA-treated plasma with 10 mM EDTA and sheared at 2400 rpm for 90 minutes at 37°C. The VWF remaining in solution after exposure to shear stress was expressed as a percentage of VWF solution in parallel samples not exposed to shear. (A) Purified VWF in LRA-treated plasma was sheared in the presence of increasing concentrations of HDL or ApoA-I. (B) Purified VWF in LRA-treated plasma was sheared in the presence of buffer (10 mM HEPES, 25 mM NaCl, 10 mM EDTA, pH 7.5), desalted serum-free medium (SFM), LRA-treated SFM, purified ApoA-I (500 μg/mL), and combinations of these reagents. (C) Purified VWF in LRA-treated plasma was sheared in the presence of boiled plasma proteins (500 μg/mL) before or after treatment with LRA. (D) SDS-gradient polyacrylamide gel electrophoresis (4% to 15%) and Gelcode blue stain (Thermo Scientific) of boiled plasma (15 μg protein) and boiled plasma after treatment with LRA (8 μg protein).
HDL decreases VWF string formation on the endothelial surface
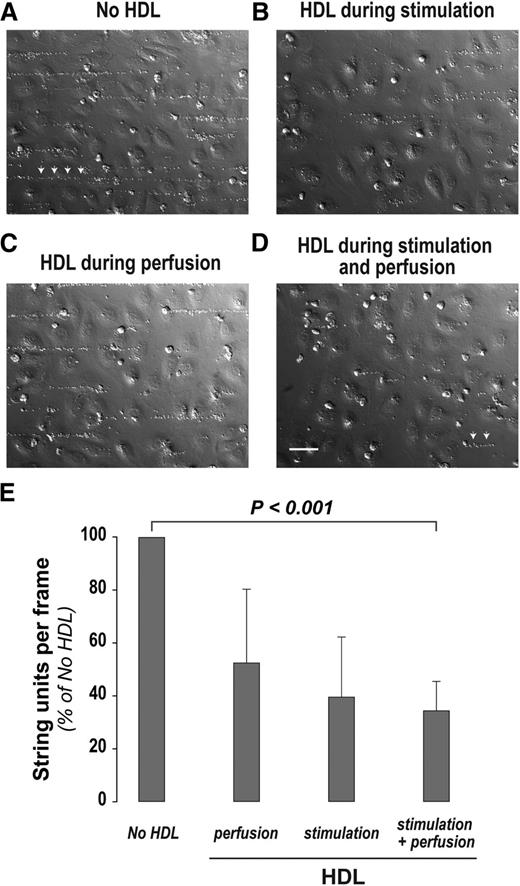
We reasoned that VWF self-association during surface adsorption is similar to or identical to the self-association that occurs on the endothelial surface when VWF forms long strands. When we activated cultured human endothelial cells grown in a flow chamber and applied fluid shear stress, hyperadhesive VWF strings formed on the endothelial surface that sometimes exceeded 50 μm in length and were capable of binding fixed platelets (Figure 3A). If HDL was present during endothelial stimulation but not during subsequent perfusion, fewer and shorter platelet-decorated strings formed (Figure 3B), an effect that might be attributed to the ability of HDL to activate endothelial nitric oxide synthase and thereby inhibit Weibel-Palade body secretion.17,18 This was not the only effect of HDL, because its presence during platelet perfusion but not during stimulation also resulted in fewer and shorter platelet-decorated strings (Figure 3C), indicating that it was interfering with VWF self-association. The presence of HDL during both stimulation and platelet perfusion further reduced the number and length of platelet-decorated strings (Figure 3D). These results, quantified and summarized in Figure 3E, showed that HDL dampened the adhesive impact of VWF by reducing its secretion and by interfering with its ability to form hyperadhesive strands.
HDL reduces VWF string assembly on the endothelial surface. Human umbilical vein endothelial cells (HUVECs) cultured in parallel-plate flow chambers were stimulated with 50 ng/mL phorbol myristate acetate (PMA) for 20 minutes at 37°C under static conditions, with or without HDL (2 mg/mL). Two mL of formaldehyde-fixed platelets (7 × 104 platelets per μL) were then perfused over the stimulated cells at a shear stress of 10 to 20 dynes/cm2 with or without HDL (200 μg/mL). The cells were then washed and fixed with paraformaldehyde. The platelet-decorated VWF strings in 10 random, nonoverlapping bright-field images for each condition were counted and measured. HUVECs were stimulated with PMA and perfused with fixed platelets (A) in the absence of HDL, (B) in the presence of HDL during stimulation but not perfusion, (C) in the presence of HDL during perfusion but not during stimulation, and (D) with HDL present during both stimulation and perfusion. (E) Quantification of VWF string units on the endothelial surface (n = 3 to 6). P value (<.001) is from Welch’s t test with Bonferroni correction for the comparison of means for no HDL vs HDL during stimulation and perfusion. Platelet-decorated VWF strings are indicated by arrows in (A) and (D). Scale bar, 50 μm (A-D).
HDL reduces VWF string assembly on the endothelial surface. Human umbilical vein endothelial cells (HUVECs) cultured in parallel-plate flow chambers were stimulated with 50 ng/mL phorbol myristate acetate (PMA) for 20 minutes at 37°C under static conditions, with or without HDL (2 mg/mL). Two mL of formaldehyde-fixed platelets (7 × 104 platelets per μL) were then perfused over the stimulated cells at a shear stress of 10 to 20 dynes/cm2 with or without HDL (200 μg/mL). The cells were then washed and fixed with paraformaldehyde. The platelet-decorated VWF strings in 10 random, nonoverlapping bright-field images for each condition were counted and measured. HUVECs were stimulated with PMA and perfused with fixed platelets (A) in the absence of HDL, (B) in the presence of HDL during stimulation but not perfusion, (C) in the presence of HDL during perfusion but not during stimulation, and (D) with HDL present during both stimulation and perfusion. (E) Quantification of VWF string units on the endothelial surface (n = 3 to 6). P value (<.001) is from Welch’s t test with Bonferroni correction for the comparison of means for no HDL vs HDL during stimulation and perfusion. Platelet-decorated VWF strings are indicated by arrows in (A) and (D). Scale bar, 50 μm (A-D).
ApoA-I prevents the association of fluid-phase VWF with immobilized VWF fibers
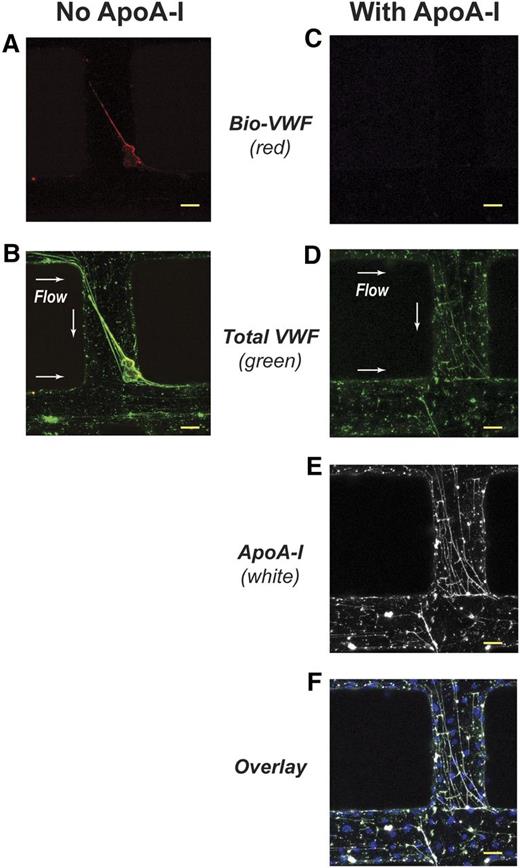
We next examined whether ApoA-I could inhibit the incorporation of fluid-phase VWF multimers into preformed VWF fibers under flow by using endothelialized in vitro microvessels.19,20 VWF secreted from stimulated microvessels self-associated into enormous fibers oriented in the direction of flow (Figure 4B). These fibers often exceeded 600 μm in length and were capable of spanning the lumen of vessels less than 300 μm in diameter. When Bio-VWF multimers were perfused through the microvessels, they attached to the immobilized VWF fibers (Figure 4A-B), another form of VWF self-association. Perfusion of ApoA-I before introducing the Bio-VWF prevented this attachment (Figure 4C). Instead, the VWF fibers became decorated with ApoA-I (Figure 4D-E). Bio-VWF was unable to displace the bound ApoA-I (Figure 4C,E).
ApoA-I prevents the binding of fluid-phase Bio-VWF to VWF fibers in synthetic microvessels. The synthetic microvessels containing HUVECs were activated with 200 ng/mL of PMA for 40 minutes at 37°C and then washed with phosphate-buffered saline (PBS) containing 6% BSA for 5 minutes. ApoA-I (500 μg/mL in SFM) or SFM was perfused over the activated microvessels for 5 minutes followed by perfusion of Bio-VWF (10 μg/mL in SFM). At the end of the experiments, the microvessels were washed with PBS containing 6% BSA for 5 minutes before staining for VWF and ApoA-I. Vessels perfused with either buffer or ApoA-I were both stained with antibody to ApoA-I. There was no signal for ApoA-I in the vessels perfused with buffer (image not shown). Secreted VWF in the microvessels self-associated to form transluminal fibers. (A) In the absence of ApoA-I, Bio-VWF (red) perfused through the activated microvessel bound to a transluminal VWF fiber, accumulating on its upstream side. (B) Colocalization of Bio-VWF (yellow) with the transluminal VWF fiber (green). (C) Absence of Bio-VWF localization in a microvessel when ApoA-I was perfused through the vessel before Bio-VWF. (D) VWF fibers (green) secreted by the activated microvessels. (E) ApoA-I (white) localization in a microvessel after perfusion. (F) Colocalization of ApoA-I (white) with VWF fibers (green). Nuclei of HUVECs are in blue. Scale bars, 50 μm (A-F). Arrows indicate direction of flow.
ApoA-I prevents the binding of fluid-phase Bio-VWF to VWF fibers in synthetic microvessels. The synthetic microvessels containing HUVECs were activated with 200 ng/mL of PMA for 40 minutes at 37°C and then washed with phosphate-buffered saline (PBS) containing 6% BSA for 5 minutes. ApoA-I (500 μg/mL in SFM) or SFM was perfused over the activated microvessels for 5 minutes followed by perfusion of Bio-VWF (10 μg/mL in SFM). At the end of the experiments, the microvessels were washed with PBS containing 6% BSA for 5 minutes before staining for VWF and ApoA-I. Vessels perfused with either buffer or ApoA-I were both stained with antibody to ApoA-I. There was no signal for ApoA-I in the vessels perfused with buffer (image not shown). Secreted VWF in the microvessels self-associated to form transluminal fibers. (A) In the absence of ApoA-I, Bio-VWF (red) perfused through the activated microvessel bound to a transluminal VWF fiber, accumulating on its upstream side. (B) Colocalization of Bio-VWF (yellow) with the transluminal VWF fiber (green). (C) Absence of Bio-VWF localization in a microvessel when ApoA-I was perfused through the vessel before Bio-VWF. (D) VWF fibers (green) secreted by the activated microvessels. (E) ApoA-I (white) localization in a microvessel after perfusion. (F) Colocalization of ApoA-I (white) with VWF fibers (green). Nuclei of HUVECs are in blue. Scale bars, 50 μm (A-F). Arrows indicate direction of flow.
ApoA-I does not compete with platelets for binding to VWF fibers
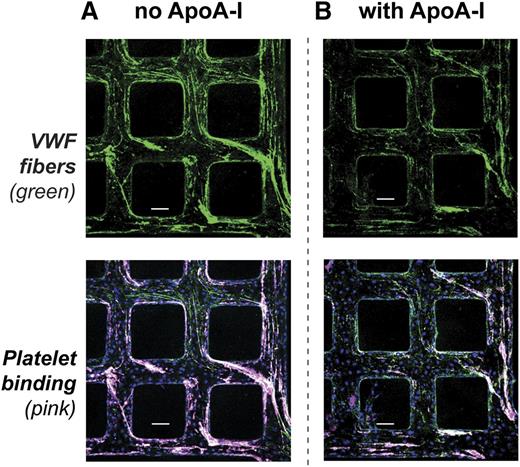
We evaluated whether ApoA-I can interfere with platelet binding to VWF by perfusing ApoA-I through activated microvessels before perfusing platelets. The VWF fibers that formed were shorter and thinner than the fibers formed in the absence of ApoA-I (Figure 5B vs 5A, upper panels). The extent of platelet accumulation was reduced to a magnitude similar to that of the reduction in VWF fibers (Figure 5B vs 5A, lower panels). The fact that the platelets still bind to ApoA-I–decorated VWF fibers suggests that diminished platelet accumulation resulted from reduced VWF fiber assembly, not from blockade of platelet adhesion sites. Consistent with this, the ratio of VWF strand fluorescence to platelet fluorescence was identical under the two conditions (supplemental Figure 7).
Platelet binding to VWF fibers in synthetic microvessels. HUVECs in synthetic microvessels were activated with 200 ng/mL PMA for 40 minutes at 37°C then washed with PBS containing 6% BSA for 5 minutes. Serum-free medium or ApoA-I (500 μg/mL) in serum-free medium was perfused over the activated microvessels for 5 minutes followed by perfusion of freshly prepared washed platelets (3 × 105 platelets per μL). (A) Without ApoA-I perfusion, washed platelets (pink) bound to VWF fibers (green) in microvessels. (B) With ApoA-I perfusion, fewer VWF fibers (green) accumulated in microvessels and fewer washed platelets (pink) bound the fibers. Scale bars, 100 μm (A-B).
Platelet binding to VWF fibers in synthetic microvessels. HUVECs in synthetic microvessels were activated with 200 ng/mL PMA for 40 minutes at 37°C then washed with PBS containing 6% BSA for 5 minutes. Serum-free medium or ApoA-I (500 μg/mL) in serum-free medium was perfused over the activated microvessels for 5 minutes followed by perfusion of freshly prepared washed platelets (3 × 105 platelets per μL). (A) Without ApoA-I perfusion, washed platelets (pink) bound to VWF fibers (green) in microvessels. (B) With ApoA-I perfusion, fewer VWF fibers (green) accumulated in microvessels and fewer washed platelets (pink) bound the fibers. Scale bars, 100 μm (A-B).
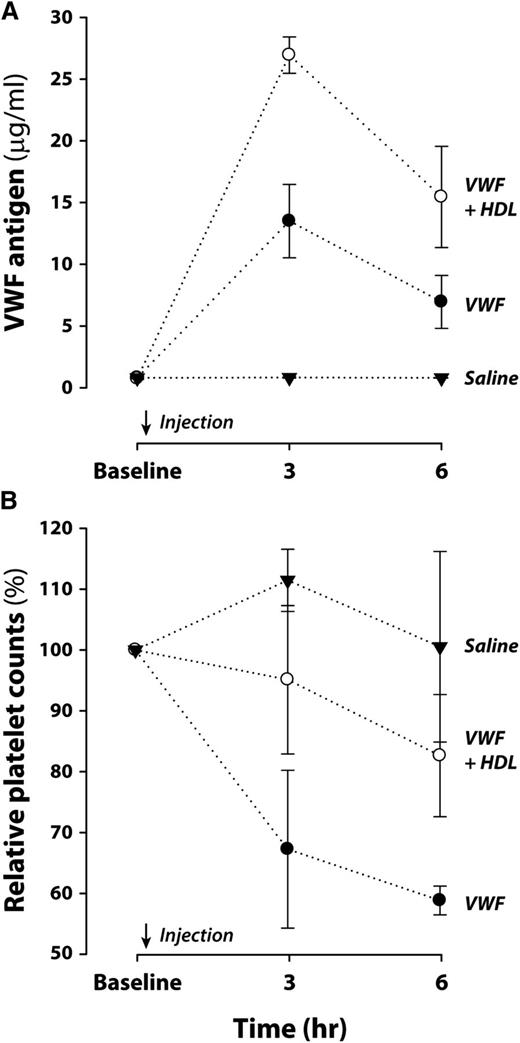
HDL dampens VWF-induced thrombocytopenia in mice
To determine whether HDL can modulate VWF function in vivo, we used a model of mild thrombotic microangiopathy induced by injection of high concentrations of human VWF into ADAMTS13-deficient mice.15 We injected 3 groups of ADAMTS13-deficient mice intravenously with purified human VWF (5 mg/kg), VWF (5 mg/kg) plus HDL (2 mg), or saline, respectively, and monitored their platelet counts. In mice injected with VWF only, the platelet counts decreased by more than 30% and 40% at 3 and 6 hours, respectively, whereas in the group injected with VWF plus HDL, platelet counts decreased only slightly (5% and 17%, respectively). The mice receiving HDL also displayed higher circulating VWF levels than mice that received only VWF (Figure 6). Schistocytosis was not significant in VWF-injected mice, and their hematocrits were not significantly different from those of mice injected with VWF plus HDL. These results suggest that HDL stabilizes VWF in circulation, prevents its self-association and binding to the vessel wall, and consequently reduces VWF-induced thrombocytopenia.
HDL decreases thrombocytopenia induced by high-dose VWF injection in ADAMTS13-deficient mice. Mice deficient in ADAMTS13 were injected with saline (n = 3), VWF (n = 4), or VWF plus HDL (n = 3). Blood was drawn from each mouse before and at 3 and 6 hours after the injection. (A) VWF levels in plasma were measured by ELISA. P values for the comparisons of means for VWF vs VWF plus HDL at 3 and 6 hours are <.001 and .05, respectively, from Welch’s t test. (B) Platelets labeled with antibody to CD41 in a Trucount tube were quantified by flow cytometry. Platelet counts at 3 and 6 hours were normalized to the counts at baseline from the same mouse and expressed as percentages of the baseline counts. P values for the comparisons of means for VWF vs VWF plus HDL at 3 and 6 hours are both <.05 from Welch’s t test.
HDL decreases thrombocytopenia induced by high-dose VWF injection in ADAMTS13-deficient mice. Mice deficient in ADAMTS13 were injected with saline (n = 3), VWF (n = 4), or VWF plus HDL (n = 3). Blood was drawn from each mouse before and at 3 and 6 hours after the injection. (A) VWF levels in plasma were measured by ELISA. P values for the comparisons of means for VWF vs VWF plus HDL at 3 and 6 hours are <.001 and .05, respectively, from Welch’s t test. (B) Platelets labeled with antibody to CD41 in a Trucount tube were quantified by flow cytometry. Platelet counts at 3 and 6 hours were normalized to the counts at baseline from the same mouse and expressed as percentages of the baseline counts. P values for the comparisons of means for VWF vs VWF plus HDL at 3 and 6 hours are both <.05 from Welch’s t test.
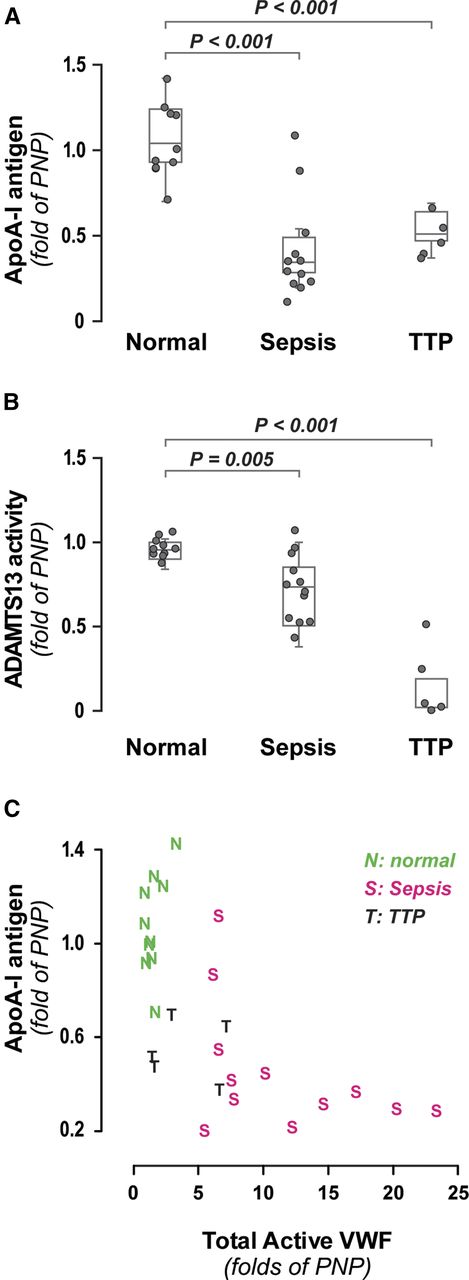
ApoA-I levels in patients with hyperadhesive VWF
The inhibitory effect of HDL or ApoA-I on VWF self-association suggests that low ApoA-I levels could favor the formation of thicker, more adhesive VWF fibers, some of which might be detected in circulation. We therefore examined the relationship between ApoA-I and hyperadhesive VWF in 2 disorders characterized by systemic endothelial activation: TTP and sepsis. We measured plasma ApoA-I concentration, ADAMTS13 activity, and total active VWF (a measure of both quantity and adhesiveness of VWF)5 in 5 patients with presumptive TTP before they underwent plasmapheresis and in 12 sepsis patients. In both groups, ApoA-I levels were significantly reduced compared with healthy controls (Figure 7A). As expected, the ADAMTS13 activity was markedly reduced in the TTP patients (Figure 7B). The patient with the highest ADAMTS13 activity (54%) was later determined to have lupus-associated thrombotic microangiopathy but was nevertheless included in the analysis for comparison. All sepsis patients had elevated total active VWF (Figure 7C); a majority (75%) also had very low levels of ApoA-I (<50%). The ADAMTS13 activity in the sepsis patients was only moderately diminished (mean, 75%) (Figure 7B), suggesting that the increased total active VWF resulted from either increased VWF secretion and/or resistance of the VWF to cleavage by ADAMTS13, which can occur by oxidation of VWF.21
ApoA-I, ADAMTS13 activity, and total active VWF levels in normal, sepsis, and TTP participants. (A) Boxplot of ApoA-I levels measured by ELISA in normal (n = 10), sepsis (n = 12), and TTP (n = 5) participants. P values for the comparisons of means, normal vs sepsis, and normal vs TTP from Dunnett’s test are both <.001. (B) Boxplot of ADAMTS13 activity levels in normal, sepsis, and presumptively diagnosed TTP participants. P values for the comparisons of means for normal vs sepsis and normal vs TTP from Dunnett’s test are .005 and <.001, respectively. (C) Distribution of ApoA-I and total active VWF levels in normal (green), sepsis (red), and TTP (black) participants. Total active VWF was determined by multiplying VWF antigen by VWF activation factor (binding to nanobody AU/VWFa-11). PNP, pooled normal plasma.
ApoA-I, ADAMTS13 activity, and total active VWF levels in normal, sepsis, and TTP participants. (A) Boxplot of ApoA-I levels measured by ELISA in normal (n = 10), sepsis (n = 12), and TTP (n = 5) participants. P values for the comparisons of means, normal vs sepsis, and normal vs TTP from Dunnett’s test are both <.001. (B) Boxplot of ADAMTS13 activity levels in normal, sepsis, and presumptively diagnosed TTP participants. P values for the comparisons of means for normal vs sepsis and normal vs TTP from Dunnett’s test are .005 and <.001, respectively. (C) Distribution of ApoA-I and total active VWF levels in normal (green), sepsis (red), and TTP (black) participants. Total active VWF was determined by multiplying VWF antigen by VWF activation factor (binding to nanobody AU/VWFa-11). PNP, pooled normal plasma.
Discussion
We report here that VWF self-association is responsible for adsorption of purified VWF onto surfaces under static conditions, shear-induced adsorption of plasma VWF onto surfaces, assembly of secreted VWF molecules into VWF strings on the endothelial surface, and incorporation of fluid-phase VWF onto endothelial VWF fibers. Importantly, we also demonstrate that VWF self-association in each of these instances is markedly attenuated by HDL or its major apolipoprotein, ApoA-I. Consistent with these in vitro results, we also show in Adamts13 knockout mice that HDL can dampen the thrombocytopenia induced by high concentrations of VWF.
Although HDL and ApoA-I were capable of blocking VWF self-association (Figures 3D and 4C), platelet adhesion was reduced only to the extent that VWF fiber formation was reduced (Figure 5B), suggesting that the sites involved in self-association or ApoA-I binding are distinct from the platelet adhesion site. One self-association site likely resides within the VWF A1A2A3 region, given that a purified A1A2A3 fragment (Asp1261-Ile1878) bound a surface coated with VWF multimers (supplemental Figure 5).
Under static conditions, several forms of ApoA-I, including ApoA-I from boiled plasma (which is likely lipid poor22 ), delipidated ApoA-I, and HDL particles, were effective in blocking VWF self-association (Figure 1 and supplemental Figures 1 and 3). The situation was different for shear-induced self-association; in that case, ApoA-I required lipid to prevent self-association (Figure 2). Consistent with this requirement, a lipid-like agent in serum-free medium, which was removed by LRA, could replace the lipid in plasma in supporting ApoA-I-mediated inhibition of VWF self-association. Another difference was that substantially higher concentrations of ApoA-I/HDL were necessary to block VWF self-association under shear conditions compared with under static conditions, indicating that the tensile force applied to VWF by shear stress exposes new self-association sites not exposed in the absence of force. Based on the HDL concentrations necessary to block VWF self-association under shear, we found that administration of HDL greatly attenuated the thrombocytopenia induced by VWF injection (Figure 7). The attenuation of thrombocytopenia correlated with elevated plasma VWF concentrations, consistent with reduced VWF self-association on the vessel wall. Taken together, these results show that HDL has a potent antithrombotic property related to regulation of VWF self-association and unrelated to its role in reverse cholesterol transport.
How ApoA-I interacts with VWF is not known. ApoA-I was able to bind VWF that was partially unfolded by shear stress and VWF strands formed as a result of self-assembly. This binding suggests that HDL/ApoA-I functions as an extracellular protein chaperone preventing the aggregation of misfolded or unfolded proteins, a function also suggested by the fact that ApoA-I binds amyloid deposits of misfolded amyloid A, immunoglobulin λ and κ chains, β2-microglobulin, transthyretin,23 and β amyloid.24
Our data indicate that the ability of VWF to support platelet adhesion on the intact endothelium and possibly also at sites of endothelial damage is influenced by a number of processes, some of them competing: the rate and extent of VWF secretion, the local shear stress, and the rates of VWF self-association, HDL binding, and ADAMTS13 cleavage. Self-association is accelerated by high concentrations of VWF and high shear stress or low levels of HDL and ADAMTS13 and is most favorable when all 4 conditions coexist.
This newly recognized VWF-specific antithrombotic property of ApoA-I/HDL has several important implications for thrombotic/vascular diseases in which VWF has been implicated. These include atherosclerosis, stroke, microvascular occlusive syndromes, and venous thrombosis. It is well described that low HDL promotes the development of atherosclerosis and that endothelial VWF recruits platelets to atherosclerosis-prone regions of the vasculature in hypercholesterolemic animals.25,26 Consistent with the role of VWF and platelets in atherosclerosis, VWF deficiency27 and acquired glycoprotein Ibα deficiency28 are protective against atherosclerosis. Furthermore, ADAMTS13 deficiency accelerates development of atherosclerosis.29 This evidence suggests that excessive VWF self-association and subsequent platelet recruitment in the setting of low HDL contribute to the development of atherosclerosis. VWF has also been implicated in the pathogenesis of stroke,30 a condition in which significant reduction in plasma ApoA-I levels has been described.31
Our data indicate that HDL/ApoA-I should also play important roles in microvascular occlusive syndromes such as TTP, sepsis, malaria, sickle cell disease, and possibly hypovolemic shock,32 and clinical data support this possibility. For example, in the 1980s, 3 patients with chronic TTP were described as having very low levels of a serum prostacyclin-binding activity,33 which was later identified as ApoA-I.34 Although treatment of plasmapheresis-resistant TTP patients with prostacyclin homologs was unsuccessful,35 the association of low ApoA-I levels with TTP has not been explained; our results suggest that the reduced ApoA-I levels in patients with TTP and sepsis may worsen these disorders. Replenishment of HDL may contribute to the effectiveness of plasmapheresis in the treatment of TTP in addition to providing ADAMTS13 and removing ADAMTS13 autoantibodies. Consistent with this notion, clinical remission of TTP after plasma exchange has been observed in the face of persistent ADAMTS13 deficiency and elevated titers of inhibitory antibodies.36 In addition to high total active VWF, low HDL and ApoA-I levels have also been described in sepsis,37 malaria,38 and sickle cell disease,39,40 suggesting that higher ApoA-I concentrations may be protective of microvascular occlusion. Consistent with this suggestion, increased mortality associated with polymicrobial sepsis was observed in ApoA-I-deficient mice.41
The ApoA-I-VWF interaction may also modulate venous thrombosis. VWF has been implicated in the pathogenesis of this disorder in epidemiologic studies42,43 and in animal models.44-46 ApoA-I protects mice from venous thrombosis, partly by suppressing endothelial activation,47 presumably reducing VWF secretion.18
In summary, we describe an important anti-adhesive, anti-thrombotic role for HDL/ApoA-I: the ability to interfere with VWF self-association and thereby reduce the length and thickness of VWF fibers, decreasing platelet adhesion. This finding connects the pathology of the microvasculature, manifested as microvascular thrombosis, with that of large vessels, manifested as atherosclerosis and arterial and venous thrombosis. This knowledge suggests a variety of new ways to treat these disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Philip G. de Groot for providing the nanobody for total active VWF determinations, Doug Bolgiano and Katherine Odem-Davis for assistance in statistical analysis, Dr Jay Heinecke for helpful discussion, and John D. Kulman for providing the pBIG expression vector.
This work was supported by grants R01HL091153 (J.A.L.), R01HL112633 (J.A.L.), R01 HL117639-01 (J.A.L.), and R21HL098672 (D.W.C.) from the National Institutes of Health, National Heart, Lung, and Blood Institute, grant HHS H30MC24049/9008441 (B.A.K.) from the Department of Health and Human Services, grants AHA12SDG9230006 (Y.Z.) and AHA09GRNT2230070 (D.W.C.) from the American Heart Association, and institutional funds from Bloodworks Northwest.
Authorship
Contribution: D.W.C. and J.A.L. conceived the study, designed experiments, analyzed data, and wrote the manuscript; J.C. designed and performed experiments, analyzed data, and helped write the manuscript; M.L., X.F., T.B., S.P., J.L., J.H., and Y.Z. performed experiments and analyzed data; and T.R.M. and B.A.K. provided reagents and advice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dominic W. Chung, Bloodworks Research Institute, 1551 Eastlake Avenue E, Suite 100, Seattle, WA 98102; e-mail: chung@bloodworksnw.org; and José A. López, Bloodworks Research Institute, 1551 Eastlake Avenue E, Suite 100, Seattle, WA 98102, e-mail: josel@bloodworksnw.org.

![Figure 1. HDL/ApoA-I prevents VWF self-association under both static and shear conditions. (A) Boiled plasma and ApoA-I prevent VWF surface adsorption and self-association under static conditions. Purified recombinant Bio-VWF (8 μg/mL) in 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) and 2 mM CaCl2 was incubated with buffer or different proteins at 22°C for 4 hours in 1.5-mL microfuge tubes. At the end of incubations, unbound proteins remaining in solution were removed. Proteins adsorbed to the tube surfaces were sequentially eluted first with buffer containing sodium dodecyl sulfate (SDS) (2% SDS, 2 mM CaCl2, 10 mM HEPES, pH 7.4), and then with 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) elution buffer (4% CHAPS, 8 M urea, 5 mM dithiothreitol, 40 mM tris(hydroxymethyl)aminomethane [Tris], pH 9.5). Bio-VWF remaining in the fluid phase and that bound to the surface were both analyzed by western blot after electrophoresis on reducing polyacrylamide gels. (B) VWF self-association is enhanced by shear stress and prevented by HDL. Purified VWF from cryoprecipitate (7 μg/mL in 10 mM HEPES, 25 mM NaCl, 10 mM EDTA, pH 7.5), Bio-VWF in serum-free medium desalted into the buffer above at 10 μg/mL, and 50% citrated plasma in 10 mM EDTA were sheared by vortexing at 2400 rpm in a rotary mixer for 90 minutes at 37°C. VWF remaining in solution was quantified by enzyme-linked immunosorbent assay (ELISA) and expressed as a percentage of the VWF in parallel samples not exposed to shear stress (n = 3). The VWF multimer distribution was analyzed by SDS-agarose gel electrophoresis and western blotting as described in supplemental Data. (C) HDL/ApoA-I dose-dependently inhibits VWF self-association under shear stress. Samples of 50% citrated plasma in 10 mM EDTA were sheared as above in increasing concentrations of HDL and ApoA-I or in single concentrations of alpha-1-acid glycoprotein (AAG) and transthyretin (each at 500 μg/mL). VWF remaining in solution was determined by ELISA. (D) Correlation of VWF remaining in solution after exposure to shear stress with the amount of VWF–ApoA-I complex formation measured by sandwich ELISA is described in supplemental Data. BSA, bovine serum albumin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/5/10.1182_blood-2014-09-599530/4/m_637f1.jpeg?Expires=1765938814&Signature=wwnBuE1ZMnMCoGqlkh5GJE9TyXVu33QUZYu1rTi~p2~f49rx0Y98n~zfsFU2aXAZsOsYvhrexdannx601gXBSVsno~FkuKUxtHMCIXEQTs8sWrSncOop~K6kvcLBJ1Tozhk-1p0mCbgXJbO3fk6hoPCcTBN6aXGYf7eljcmeQTxc9tI6X2rbxqRPCfI-vNDiCnIl~xX5f~AvaSv1KSEB5Wk18T2aMppMlkIfxa~uupmRGohyeh0p~A2-73hc8yFWWPaXMqX9IQ-KuQDRY7C47bsIKgr6LqozUU0UAjfgX8NCyGxzQ8ajjzsDOpvsU3bRKUO4-~Ph~l60B5zeIncF1w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal