Key Points
An in vivo model of MDS displays time-dependent defects in HSPCs and in microenvironmental populations.
Normalization of the marrow microenvironment alters disease progression and transformation and improves hematopoietic function.
Abstract
In vitro evidence suggests that the bone marrow microenvironment (BMME) is altered in myelodysplastic syndromes (MDSs). Here, we study the BMME in MDS in vivo using a transgenic murine model of MDS with hematopoietic expression of the translocation product NUP98-HOXD13 (NHD13). This model exhibits a prolonged period of cytopenias prior to transformation to leukemia and is therefore ideal to interrogate the role of the BMME in MDS. In this model, hematopoietic stem and progenitor cells (HSPCs) were decreased in NHD13 mice by flow cytometric analysis. The reduction in the total phenotypic HSPC pool in NHD13 mice was confirmed functionally with transplantation assays. Marrow microenvironmental cellular components of the NHD13 BMME were found to be abnormal, including increases in endothelial cells and in dysfunctional mesenchymal and osteoblastic populations, whereas megakaryocytes were decreased. Both CC chemokine ligand 3 and vascular endothelial growth factor, previously shown to be increased in human MDS, were increased in NHD13 mice. To assess whether the BMME contributes to disease progression in NHD13 mice, we performed transplantation of NHD13 marrow into NHD13 mice or their wild-type (WT) littermates. WT recipients as compared with NHD13 recipients of NHD13 marrow had a lower rate of the combined outcome of progression to leukemia and death. Moreover, hematopoietic function was superior in a WT BMME as compared with an NHD13 BMME. Our data therefore demonstrate a contributory role of the BMME to disease progression in MDS and support a therapeutic strategy whereby manipulation of the MDS microenvironment may improve hematopoietic function and overall survival.
Introduction
Myelodysplastic syndromes (MDSs) are malignant hematopoietic stem cell (HSC) disorders characterized by ineffective hematopoiesis resulting in blood cytopenias and the risk of clonal evolution to acute myelogenous leukemia.1 In vitro stromal abnormalities in MDS2-11 have suggested the possibility that the bone marrow microenvironment (BMME) may contribute to disease progression and may therefore be a valuable therapeutic target. However, the lack of examination of the BMME in a robust in vivo model of MDS has limited progress in understanding the reciprocal interactions between abnormal MDS hematopoietic cells and the BMME. Although considerable efforts have been made to study human MDS samples and develop xenotransplantation models, murine models of MDS recapitulate the histologic and cytological features of human MDS and thus represent a valuable research tool for understanding disease mechanisms and identifying potential therapeutic targets.12 Specifically, mice expressing the hematopoietic compartment-specific, Vav-driven NUP98-HOXD13 fusion transgene, first identified in a patient with MDS, exhibit multilineage cytopenias and dysplasia, along with the potential to transform to acute leukemias.13-15 Another challenge in understanding the MDS-BMME interactions is that past reports have defined the BMME in an inconsistent manner. Recently, use of genetic models has permitted the identification of BMME populations critical for HSC support.16-18 Whether these populations are altered in vivo in established models for MDS has not been studied.
Because stimulation of the BMME in murine models can improve hematopoietic recovery from radiation19-21 and chemotherapy,22 and can inhibit progression of malignancy,23 the identification of targetable components of the BMME in MDS may allow for their beneficial therapeutic manipulation. To definitively demonstrate the potential efficacy of targeting the BMME in MDS, we used the Vav-NUP98-HOXD13 (NHD13) transgenic model and investigated if the BMME contributes to marrow failure and conversion to leukemia.
Methods
Mice
All mice were maintained within the Vivarium facility at the University of Rochester School of Medicine and Dentistry in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Mice expressing the transgene NUP98-HOXD13 (under the control of the vav oncogene regulatory elements) and the Ly5.2 allotype of CD45 (45.2) on a C57BL/6 background were purchased from Jackson Laboratory. Progeny were genotyped for presence of NHD13 with standard polymerase chain reactions.
See supplemental Materials and Methods (available on the Blood Web site) for additional methods.
Results
NHD13 mice recapitulate MDS and develop a progressive quantitative defect in the hematopoietic stem and progenitor cell (HSPC) pool
Consistent with prior reports, NHD13 mice developed progressive cytopenias and macrocytosis (Figure 1A-D) with blood and marrow dysplasia (Figure 1E-F and data not shown), meeting the Bethesda criteria for MDS by 15 to 20 weeks from birth. Both peripheral blood cell counts and flow cytometric analysis of peripheral blood from 17- to 18-week-old mice showed myeloid skewing within the white blood cell (WBC) pool of NHD13 mice compared with WT littermates (Figure 1G). NHD13 mice demonstrated leukemic transformation and mortality occurring by 32 weeks of age (Figure 1H). Transformation was confirmed by high peripheral WBC counts, massive splenomegaly, and presence of marrow and circulating blasts (supplemental Figure 1A-B). Flow cytometric phenotypic analysis of spleen cells in 1 example showed high expression of CD3e and B220 and low expression of CD11b, suggestive of an acute lymphoblastic leukemia (supplemental Figure 1C). Although patients with MDS develop acute myelogenous leukemia more commonly, lymphoid malignancies are more common in mice and have been reported to occur in the NHD13 model.13 We confirmed that the acute leukemia was transplantable, as demonstrated by >90% donor engraftment in the marrow, spleen, and peripheral blood of nonirradiated WT recipient mice at 3 weeks posttransplant (Figure 1J). Moreover, mice transplanted with the transformed NHD13 leukemic spleen cells demonstrated splenomegaly (Figure 1K) and blasts in the peripheral blood (Figure 1L) with mortality by 3 weeks posttransplant (supplemental Figure 1E), consistent with leukemic development. Because our goal was to define the role of the HSPC microenvironment in MDS, we focused the majority of our studies at 17 to 25 weeks of age, when cytopenias are evident but leukemia is rare.
NHD13 mice develop cytopenias, macrocytosis, and myeloid dysplasia, with increased rate of progression to leukemia or death. (A-D) Male and female NHD13 (blue diamond) and wild-type (WT; gray square) littermates were followed with serial complete blood count measurements (WT, n = 2-12; NHD13, n = 3-14). Data from leukemic animals censored at the first time point at which they were noted to be leukemic. Results analyzed for statistical significance using 2-way analysis of variance (ANOVA). P = .0025 for granulocytes; P < .0001 for all other parameters. (E) Normal neutrophil in the peripheral blood of a WT mouse. (×1000 oil immersion). (F) Dysplastic neutrophil in the peripheral blood of representative NHD13 mouse (×1000 oil immersion). (G) Flow cytometric analysis of mononuclear cells in peripheral blood (myeloid, CD11b+; lymphoid, CD3e+ or B220+). (H) Survival curves showing progression to leukemia or death in NHD13 or WT mice (log-rank [Mantel-Cox] test: P = .09). (I) Schematic of transplant setup. Spleen cells (1 x 106) from NHD13 mouse (CD45.2) noted to have high peripheral WBC counts and massive splenomegaly were transplanted to WT CD45.1 recipients. (J) Leukemic donor cells demonstrated engraftment of >90% in recipients at 3 weeks posttransplant. (K) Representative spleens from nontransplanted WT (left) and recipients of NHD13 cells (right) demonstrating splenomegaly consistent with leukemic development at 3 weeks posttransplant. (L) Representative peripheral blood smears from recipients showing blasts consistent with leukemic development at 3 weeks posttransplant.
NHD13 mice develop cytopenias, macrocytosis, and myeloid dysplasia, with increased rate of progression to leukemia or death. (A-D) Male and female NHD13 (blue diamond) and wild-type (WT; gray square) littermates were followed with serial complete blood count measurements (WT, n = 2-12; NHD13, n = 3-14). Data from leukemic animals censored at the first time point at which they were noted to be leukemic. Results analyzed for statistical significance using 2-way analysis of variance (ANOVA). P = .0025 for granulocytes; P < .0001 for all other parameters. (E) Normal neutrophil in the peripheral blood of a WT mouse. (×1000 oil immersion). (F) Dysplastic neutrophil in the peripheral blood of representative NHD13 mouse (×1000 oil immersion). (G) Flow cytometric analysis of mononuclear cells in peripheral blood (myeloid, CD11b+; lymphoid, CD3e+ or B220+). (H) Survival curves showing progression to leukemia or death in NHD13 or WT mice (log-rank [Mantel-Cox] test: P = .09). (I) Schematic of transplant setup. Spleen cells (1 x 106) from NHD13 mouse (CD45.2) noted to have high peripheral WBC counts and massive splenomegaly were transplanted to WT CD45.1 recipients. (J) Leukemic donor cells demonstrated engraftment of >90% in recipients at 3 weeks posttransplant. (K) Representative spleens from nontransplanted WT (left) and recipients of NHD13 cells (right) demonstrating splenomegaly consistent with leukemic development at 3 weeks posttransplant. (L) Representative peripheral blood smears from recipients showing blasts consistent with leukemic development at 3 weeks posttransplant.
Hematopoietic progenitor population heterogeneity and decreased HSC populations in the NHD13 model
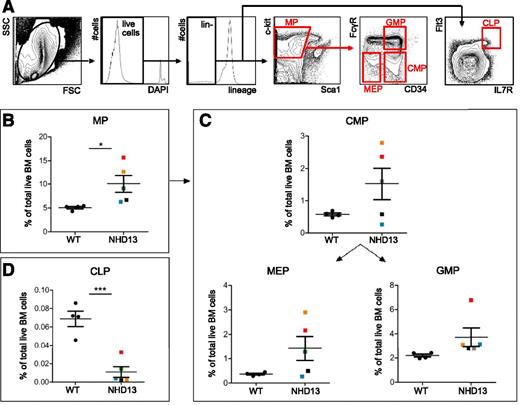
We examined the hematopoietic progenitor compartment at 25 weeks from birth. Notably, impaired function of NHD13 myeloid progenitors has been previously reported in NHD13 mice in vitro based on colony-forming unit (CFU) assays.15 Although the marrow cellularity was similar between the NHD13 and WT mice (9.5 × 107 ± 5.9 × 106 vs 9.6 × 107 ± 1.6 × 106 cells from the total of bilateral femora, tibiae, and pelvic bones), the myeloid progenitor pool was significantly increased (Figure 2B), as would be expected in MDS. Notably, within the myeloid progenitor population in NHD13 marrow compared with WT marrow, there was significant variability in the frequency of common myeloid progenitors, megakaryocyte-erythroid progenitors, and granulocyte-monocyte progenitors (Figure 2C). Phenotypic common lymphoid progenitors were diminished in 25-week-old NHD13 mice (as compared with WT littermates) (Figure 2D). When NHD13 mice were examined on an individual basis, they demonstrated wide variations in the differential expansion of myeloid progenitor subsets, in spite of the use of a transgenic model, suggesting that defects in the hematopoietic progenitor pools are heterogeneous in MDS.
Heterogeneity of the phenotypic myeloid progenitor pool in NHD13 mice. Bone marrow (BM) was harvested from 25-week-old male and female NHD13 (n = 5) and WT (n = 4) littermates via the crushing technique. BM was analyzed for progenitor frequencies via flow cytometry. (A) Representative flow cytometric gating schema on the whole BM from a 25-week-old WT mouse in which common lymphoid progenitors (CLPs) (Lin−/Flt3+/IL7R+) and myeloid progenitors (MPs) (Lin−/c-Kit+/Sca1−) are a subset of the lineage-negative (live) cell population, the latter of which can be divided into common myeloid progenitors (CMPs) (FcγR−/CD34+), megakaryocyte-erythrocyte progenitors (MEPs) (FcγR−/CD34−), and granulocyte-macrophage progenitors (GMPs) (FcγR+/CD34+). (B-D) Quantification of progenitor populations in WT compared with NHD13 littermates. In all graphs, each dot represents an individual mouse, and color denotes the same individual mouse in each gate. *P < .05; ***P < .001.
Heterogeneity of the phenotypic myeloid progenitor pool in NHD13 mice. Bone marrow (BM) was harvested from 25-week-old male and female NHD13 (n = 5) and WT (n = 4) littermates via the crushing technique. BM was analyzed for progenitor frequencies via flow cytometry. (A) Representative flow cytometric gating schema on the whole BM from a 25-week-old WT mouse in which common lymphoid progenitors (CLPs) (Lin−/Flt3+/IL7R+) and myeloid progenitors (MPs) (Lin−/c-Kit+/Sca1−) are a subset of the lineage-negative (live) cell population, the latter of which can be divided into common myeloid progenitors (CMPs) (FcγR−/CD34+), megakaryocyte-erythrocyte progenitors (MEPs) (FcγR−/CD34−), and granulocyte-macrophage progenitors (GMPs) (FcγR+/CD34+). (B-D) Quantification of progenitor populations in WT compared with NHD13 littermates. In all graphs, each dot represents an individual mouse, and color denotes the same individual mouse in each gate. *P < .05; ***P < .001.
The phenotypic HSPC compartment within the BM, defined as cells that are negative for lineage markers and positive for cKit and Sca1 (LSK), is enriched for HSCs but is very heterogeneous, including both quiescent HSCs and the short-lived multipotent progenitors (MPPs). Notably, MPPs were recently demonstrated to support the daily regenerative needs of the hematopoietic system.24 Previous studies in NHD13 mice showed that the LSK compartment as a whole is decreased14 and demonstrated an increase in apoptosis,15 whereas hematopoietic differentiation is impaired.25 To better define HSPC populations in NHD13 mice, we performed flow cytometric analysis of whole BM, and by 20 weeks of age, we quantified a significant decrease in all phenotypic HSPC subsets (Figure 3A and supplemental Figure 2). Consistent with prior reports, BM cellularity was unchanged in NHD13 mice compared with WT mice (1.3 × 107 ± 1.3 × 106 vs 1.4 × 107 ± 1.5 × 106 cells per 2 hind limbs). In juvenile NHD13 mice, there were no significant differences in LSK cells, MPPs, and long-term HSCs (supplemental Figure 2). However, there was an age-dependent decline in the NHD13 total HSPC pool, including MPPs, short-term HSCs, and long-term HSCs (supplemental Figure 2).
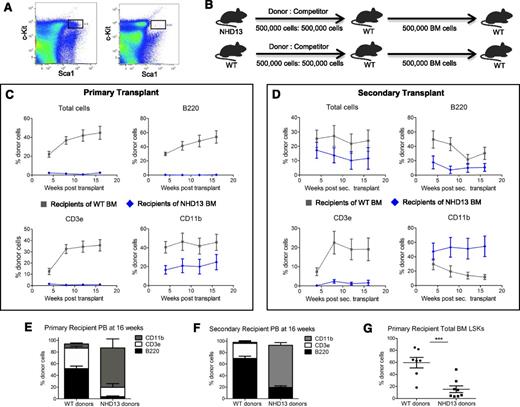
Phenotypic and functional loss of HSPC pool in NHD13 mice. (A) Representative flow plots gating for LSKs in the marrow of 5 female 20-week-old WT mice (concatenated data; left) and 5 female 20-week-old NHD13 mice (concatenated data; right). (B) Schematic of competitive repopulation assay. Whole BM from 4 22-week-old NHD13 or WT littermate donors was transplanted in a 1:1 ratio with normal WT whole BM into each of 2 lethally irradiated WT recipients. After harvesting primary recipient BM at 16 weeks posttransplant, 500 000 whole BM cells were transplanted into lethally irradiated secondary WT CD45.1 recipients. (C) Serial blood flow cytometric analysis was performed at 4-week intervals. Mice transplanted with NHD13 marrow (n = 8) had decreased donor-derived HSPC function compared with those transplanted with WT marrow (n = 7) as measured by percent of donor cells in the peripheral blood. Results analyzed for statistical significance using 2-way ANOVA with point matching; P < .01 for all parameters. Bonferroni posttests significant for all except CD11b curves. (D) Serial blood flow cytometric analysis in secondary transplant recipients. Primary donor NHD13 cells contributed proportionately less to circulating B and T cells in secondary recipients (P < .0001 by 2-way ANOVA with point matching) and more to circulating myeloid cells (P < .0001). (E) At 16 weeks post–primary transplant, percent lineage contribution to CD45.2 blood cells is shown from NHD13 (n = 7) and WT (n = 7). (F) Sixteen weeks post–secondary transplant, percent lineage contribution to CD45.2 blood cells is shown from NHD13 (n = 8) and WT (n = 7). (G) Donor contribution to LSK pool in the BM of primary recipients harvested 16 weeks posttransplant. Results analyzed for significance via Student t test. Each dot represents an individual mouse. ***P < .001.
Phenotypic and functional loss of HSPC pool in NHD13 mice. (A) Representative flow plots gating for LSKs in the marrow of 5 female 20-week-old WT mice (concatenated data; left) and 5 female 20-week-old NHD13 mice (concatenated data; right). (B) Schematic of competitive repopulation assay. Whole BM from 4 22-week-old NHD13 or WT littermate donors was transplanted in a 1:1 ratio with normal WT whole BM into each of 2 lethally irradiated WT recipients. After harvesting primary recipient BM at 16 weeks posttransplant, 500 000 whole BM cells were transplanted into lethally irradiated secondary WT CD45.1 recipients. (C) Serial blood flow cytometric analysis was performed at 4-week intervals. Mice transplanted with NHD13 marrow (n = 8) had decreased donor-derived HSPC function compared with those transplanted with WT marrow (n = 7) as measured by percent of donor cells in the peripheral blood. Results analyzed for statistical significance using 2-way ANOVA with point matching; P < .01 for all parameters. Bonferroni posttests significant for all except CD11b curves. (D) Serial blood flow cytometric analysis in secondary transplant recipients. Primary donor NHD13 cells contributed proportionately less to circulating B and T cells in secondary recipients (P < .0001 by 2-way ANOVA with point matching) and more to circulating myeloid cells (P < .0001). (E) At 16 weeks post–primary transplant, percent lineage contribution to CD45.2 blood cells is shown from NHD13 (n = 7) and WT (n = 7). (F) Sixteen weeks post–secondary transplant, percent lineage contribution to CD45.2 blood cells is shown from NHD13 (n = 8) and WT (n = 7). (G) Donor contribution to LSK pool in the BM of primary recipients harvested 16 weeks posttransplant. Results analyzed for significance via Student t test. Each dot represents an individual mouse. ***P < .001.
Dysfunction of HSPCs in NHD13 mice
To investigate whether the loss in the marrow HSPC pool of NHD13 mice was accompanied by decreased HSC function, we performed competitive transplant assays with donor NHD13 or WT littermate marrow and competitor WT marrow in primary WT recipients. At 16 weeks post–primary transplant, recipients were euthanized and whole BM was harvested and secondarily transplanted into irradiated WT recipients (Figure 3B). NHD13 donor contribution was severely diminished compared with WT donors (Figure 3C), corresponding with the decrease in the observed phenotypic MPPs and short-term HSCs (supplemental Figure 2). Additionally, we observed marked myeloid skewing in recipients of NHD13 donor marrow as compared with the WT donor marrow (Figure 3E), a phenotype associated with HSC aging.26-28 In secondary transplant recipients, defective NHD13 donor cell engraftment was again noted other than for the myeloid lineage, where NHD13 donor cell contribution was higher than WT donor contribution (Figure 3D). More severe myeloid skewing of the NHD13 donor marrow was again observed in the secondary transplant (Figure 3F). This result may explain the previously reported growth advantage of NHD13 cells over WT cells in competitive transplant experiments.14 Flow cytometric analysis of the marrow HSPC pool at 16 weeks post–primary transplant demonstrated that donor contribution to the HSPC pool was decreased in the recipients of NHD13 donor marrow compared with WT donor marrow, again demonstrating dysfunction of HSPCs from NHD13 mice (Figure 3G).
The phenotypic heterogeneity we first observed in hematopoietic progenitors was also noted in the recipients of transplanted marrow, although this variability was decreased between recipients of the same donor (supplemental Figure 3). This heterogeneity suggests that further analysis of the BMME should be investigated cautiously, ideally via exposure of the same hematopoietic system to different (WT vs NHD13) microenvironments.
Increased endothelial cell populations and decreased megakaryocytes in NHD13 mice
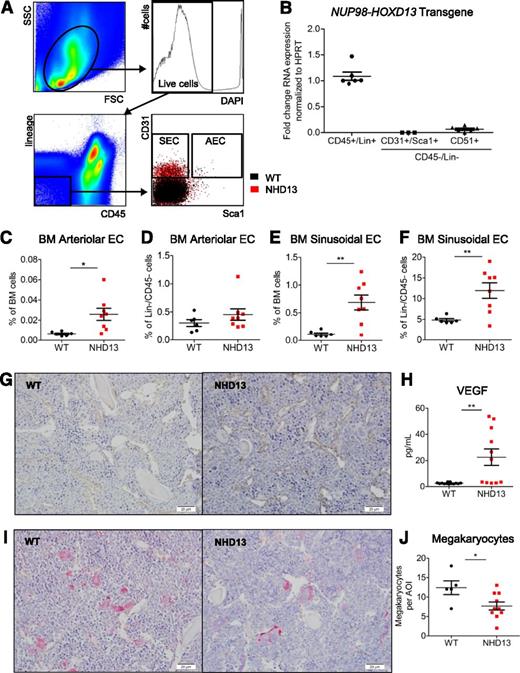
Because HSCs were decreased in NHD13 mice, we hypothesized that the MDS BMME of NHD13 mice would have an impaired ability to support HSPCs. To define components of the NHD13 BMME that may be abnormal, we first used flow cytometry to quantify marrow stromal populations (Figure 4A).29 We confirmed specific expression of the NUP98-HOXD13 transgene in hematopoietic cells and not in endothelial and stromal/osteoblastic cell populations (Figure 4B). NHD13 mice (18 to 23 weeks old) had increased Lin−/CD31+/Sca1+ and Lin−/CD31+/Sca1− populations enriched for AECs and SECs, respectively (Figure 4C-F). We also note a qualitative increase in endothelial structures in histologic sections of NHD13 mice compared with WT (Figure 4G). Notably, the levels of vascular endothelial growth factor (VEGF) were increased in the marrow of NHD13 mice compared with WT (Figure 4H). Therefore, in this model of MDS there are increases in marrow endothelial populations compared with age-matched WT littermates. Because megakaryocytes contribute to HSC regulation, we quantified GP1bβ+ cells in histologic sections (Figure 4I) and found them to be decreased in NHD13 mice compared with WT (Figure 4J). Therefore, both endothelial and megakaryocyte abnormalities could contribute to the HSC dysfunction in this murine model of MDS.
Increased endothelial cell populations and decreased megakaryocytes in NHD13 mice. (A) Flow cytometry gating strategy used to identify BM Lin−/CD45− nonhematopoietic cells, Lin−/CD45−/CD31+/Sca1+ cells enriched for arteriolar endothelial cells (AECs), and Lin−/CD45−/CD31+/Sca1− cells enriched for sinusoidal endothelial cells (SECs). The first 3 plots represent WT mice, and the last plots show overlay of populations from concatenated NHD13 (red) and WT (black) mice data. (B) Expression of NUP98/HOXD13 transgene in hematopoietic, endothelial, and stromal cells. (C-D) Frequency of AECs within total BM pool (C) and Lin−/CD45− nonhematopoietic pool (D) of NHD13 and WT mice. (E-F) Frequency SECs within total BM pool (E) and Lin−/CD45− nonhematopoietic pool (F) of NHD13 and WT mice. (G) Endomucin immunohistochemistry for metaphyseal region of representative WT and NHD13 mice. Bars represent 20 µm. (H) Peripheral blood serum VEGF level measured by Luminex xMAP assay. (I) GP1bβ immunohistochemistry for metaphyseal region of representative WT and NHD13 mouse. Bars represent 20 µm. (J) Quantification of GP1bβ+ megakaryocyte numbers per area of interest (AOI) in femora of WT and NHD13 mice. For all graphs, *P < .05; **P < .01. Each dot represents an individual mouse; mean and standard error of the mean are shown.
Increased endothelial cell populations and decreased megakaryocytes in NHD13 mice. (A) Flow cytometry gating strategy used to identify BM Lin−/CD45− nonhematopoietic cells, Lin−/CD45−/CD31+/Sca1+ cells enriched for arteriolar endothelial cells (AECs), and Lin−/CD45−/CD31+/Sca1− cells enriched for sinusoidal endothelial cells (SECs). The first 3 plots represent WT mice, and the last plots show overlay of populations from concatenated NHD13 (red) and WT (black) mice data. (B) Expression of NUP98/HOXD13 transgene in hematopoietic, endothelial, and stromal cells. (C-D) Frequency of AECs within total BM pool (C) and Lin−/CD45− nonhematopoietic pool (D) of NHD13 and WT mice. (E-F) Frequency SECs within total BM pool (E) and Lin−/CD45− nonhematopoietic pool (F) of NHD13 and WT mice. (G) Endomucin immunohistochemistry for metaphyseal region of representative WT and NHD13 mice. Bars represent 20 µm. (H) Peripheral blood serum VEGF level measured by Luminex xMAP assay. (I) GP1bβ immunohistochemistry for metaphyseal region of representative WT and NHD13 mouse. Bars represent 20 µm. (J) Quantification of GP1bβ+ megakaryocyte numbers per area of interest (AOI) in femora of WT and NHD13 mice. For all graphs, *P < .05; **P < .01. Each dot represents an individual mouse; mean and standard error of the mean are shown.
Increased dysfunctional osteoblastic lineage cells (OBCs) within the BM of NHD13 mice
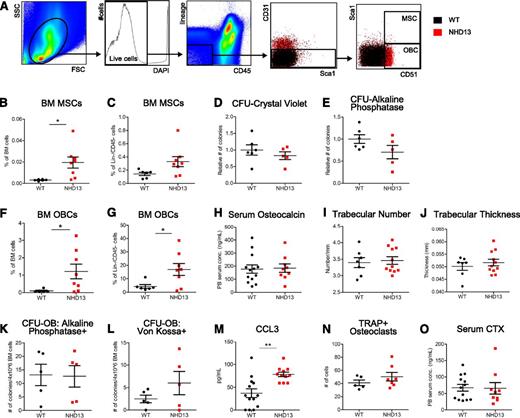
We next focused on the mesenchymal/osteoblastic lineage. At 18 to 23 weeks of age, NHD13 mice had increased phenotypic multipotent stromal cells (MSCs; Figure 5B-C), which are thought to give rise to OBCs; however, functional MSCs were not increased (Figure 5D-E). NHD13 mice also demonstrated increased OBCs within the bulk BM and within the nonhematopoietic (Lin−/CD45−) fraction of the BM compared with WT mice (Figure 5F-G). Because osteoblasts synthesize and release osteocalcin into blood during bone synthesis, serum osteocalcin levels correlate with functional osteoblasts. Serum osteocalcin levels were unchanged in 23-week-old NHD13 mice compared with WT mice (Figure 5H). Because osteoblasts are normally involved in bone synthesis, an expansion of functional osteolineage cells in NHD13 mice may result in an altered bone phenotype. Micro–computed tomography quantification of the femora (Figure 5I-J) and tibiae (data not shown) of NHD13 and WT mice showed no difference in trabecular bone thickness and number. Moreover, there were no increases in osteoprogenitors, as quantified by CFU osteoblast (CFU-OB) assays (Figure 5K-L).Together, these data suggest that the expanded osteolineage cells are not functional bone-forming cells. Because we and others have previously reported that CC chemokine ligand 3 (CCL3) is a modulator of the BMME in other murine models of hematopoietic malignancies,29,30 serum CCL3 levels were measured and found to be significantly increased in marrow of NHD13 compared with WT littermates (Figure 5M). As bone formation and resorption in the skeleton are balanced processes to maintain skeletal homeostasis, osteoclastic function was also examined in NHD13 mice. Osteoclasts, identified histologically as TRAP+ endosteal cells, were unchanged in NHD13 compared with WT mice (Figure 5N). Furthermore, serum levels of CTX, released by osteoclastic degradation of type I collagen during bone resorption, were similar in NHD13 compared with WT mice at 23 weeks of age (Figure 5N). Thus, similar osteoclastic numbers and activity in NHD13 and WT mice suggest that the absence of bone changes in the setting of increased osteolineage cells is not because of alterations in osteoclastic function.
Expansion of multipotent stromal and OBCs in NHD13 mice that are nonfunctional. (A) Flow cytometry gating strategy used to identify BM Lin−/CD45− nonhematopoietic cells, Lin−/CD45−/CD31−/CD51+/Sca1− OBCs, and Lin−/CD45−/CD31−/CD51+/Sca1+ MSCs. The first 3 plots represent WT mice, and the last 2 plots show overlay of populations from concatenated NHD13 (red) and WT mice (black) data. (B-C) Frequency of MSCs within total BM pool (B) and Lin−/CD45− nonhematopoietic pool (C) of NHD13 and WT mice. (D-E) Number of total CFU fibroblasts (identified by staining with crystal violet) (D) and alkaline phosphatase–positive CFU fibroblasts (E) from 1 × 106 BM cells after 10 to 14 days in culture. Data from 2 separate experiments and NHD13 data points represent mean of triplicate cultures normalized to mean of WT group. (F-G) Frequency of OBCs within total BM pool (F) and Lin−/CD45− nonhematopoietic pool (G) of NHD13 and WT mice. (H) Peripheral blood serum osteocalcin levels measured by enzyme-linked immunosorbent assay. (I-J) Femoral trabecular number (I) and thickness (J) as measured by micro–computed tomography. (K-L) Number of alkaline phosphatase–positive CFU-OBs (K) and Von Kossa–positive bone nodules (L) formed by 4 × 106 BM cells after 17 days of culture in mineralization media. Each data point represents mean number of CFU-OBs from triplicate cultures. (M) Peripheral blood serum CCL3 levels measured by Luminex xMAP assay. (N) Number of histologically identified osteoclasts as TRAP+ endosteal-associated cells. (O) Peripheral blood serum C-telopeptides (CTX) levels measured by enzyme-linked immunosorbent assay. All mice used in analyses are 20 ± 3 weeks of age. For all graphs, *P < .05; **P < .01; mean and standard error of the mean are shown.
Expansion of multipotent stromal and OBCs in NHD13 mice that are nonfunctional. (A) Flow cytometry gating strategy used to identify BM Lin−/CD45− nonhematopoietic cells, Lin−/CD45−/CD31−/CD51+/Sca1− OBCs, and Lin−/CD45−/CD31−/CD51+/Sca1+ MSCs. The first 3 plots represent WT mice, and the last 2 plots show overlay of populations from concatenated NHD13 (red) and WT mice (black) data. (B-C) Frequency of MSCs within total BM pool (B) and Lin−/CD45− nonhematopoietic pool (C) of NHD13 and WT mice. (D-E) Number of total CFU fibroblasts (identified by staining with crystal violet) (D) and alkaline phosphatase–positive CFU fibroblasts (E) from 1 × 106 BM cells after 10 to 14 days in culture. Data from 2 separate experiments and NHD13 data points represent mean of triplicate cultures normalized to mean of WT group. (F-G) Frequency of OBCs within total BM pool (F) and Lin−/CD45− nonhematopoietic pool (G) of NHD13 and WT mice. (H) Peripheral blood serum osteocalcin levels measured by enzyme-linked immunosorbent assay. (I-J) Femoral trabecular number (I) and thickness (J) as measured by micro–computed tomography. (K-L) Number of alkaline phosphatase–positive CFU-OBs (K) and Von Kossa–positive bone nodules (L) formed by 4 × 106 BM cells after 17 days of culture in mineralization media. Each data point represents mean number of CFU-OBs from triplicate cultures. (M) Peripheral blood serum CCL3 levels measured by Luminex xMAP assay. (N) Number of histologically identified osteoclasts as TRAP+ endosteal-associated cells. (O) Peripheral blood serum C-telopeptides (CTX) levels measured by enzyme-linked immunosorbent assay. All mice used in analyses are 20 ± 3 weeks of age. For all graphs, *P < .05; **P < .01; mean and standard error of the mean are shown.
Replacement of the MDS BMME with a WT BMME mitigates transformation to leukemia and death in recipients of NHD13 marrow
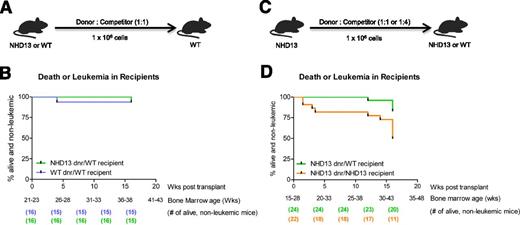
We next examined if exposure of NHD13 marrow to a WT BMME would change mortality and transformation to leukemia. Notably, NHD13 marrow donors were at least 20 weeks old at time of transplant; therefore, based on our data (Figure 1H), we would expect significant death and/or leukemia in recipients of NHD13 marrow at the later time points of the transplant. Restoration of the BMME using transplantation reversed the increased mortality and risk for leukemic transformation associated with the NUP98-HOXD13 transgene (Figure 6B), demonstrating that disease progression in NHD13 mice can be mitigated via normalization of the BMME.
Impact of the BMME on survival and leukemia. (A) Schematic of transplants into WT recipients. (B) In WT recipients, survival curves indicate mitigation of progression to leukemia or death. Data collected from 8 separate experiments. Log-rank (Mantel-Cox) test: P = .98. The BM age demarcated on the x-axis is indicative of primary donor BM age based on date of birth of the primary donor. No leukemia was diagnosed in these groups. (C) Schematic of competitive repopulation assay in which marrow from an NHD13 mouse was competitively transplanted into either irradiated NHD13 or WT recipients. (D) Survival curves showing progression to leukemia or death in primary WT recipients of competitively transplanted NHD13 marrow and in primary NHD13 recipients of competitively transplanted NHD13 marrow. Data collected from 6 separate experiments. Leukemia was formally diagnosed in 1 WT recipient and 4 NHD13 recipients. The same donor/competitor ratios were present in both experimental groups. Log-rank (Mantel-Cox) test: P = .01. In all transplants, competitor marrow was 6- to 8-week-old WT marrow. The BM age demarcated on the x-axis is indicative of primary donor BM age based on date of birth of the primary donor.
Impact of the BMME on survival and leukemia. (A) Schematic of transplants into WT recipients. (B) In WT recipients, survival curves indicate mitigation of progression to leukemia or death. Data collected from 8 separate experiments. Log-rank (Mantel-Cox) test: P = .98. The BM age demarcated on the x-axis is indicative of primary donor BM age based on date of birth of the primary donor. No leukemia was diagnosed in these groups. (C) Schematic of competitive repopulation assay in which marrow from an NHD13 mouse was competitively transplanted into either irradiated NHD13 or WT recipients. (D) Survival curves showing progression to leukemia or death in primary WT recipients of competitively transplanted NHD13 marrow and in primary NHD13 recipients of competitively transplanted NHD13 marrow. Data collected from 6 separate experiments. Leukemia was formally diagnosed in 1 WT recipient and 4 NHD13 recipients. The same donor/competitor ratios were present in both experimental groups. Log-rank (Mantel-Cox) test: P = .01. In all transplants, competitor marrow was 6- to 8-week-old WT marrow. The BM age demarcated on the x-axis is indicative of primary donor BM age based on date of birth of the primary donor.
Accelerated transformation of NHD13 marrow to leukemia and death in NHD13 compared with WT BMME
To better define if the NHD13 BMME contributes to disease progression in NHD13 mice, we transplanted NHD13 donor marrow with WT competitor marrow into age-matched NHD13 and WT littermates, as shown in Figure 6C. We used a single NHD13 donor for each experiment, so that for each donor, engraftment in WT or NHD13 recipient littermates could be compared. This allowed assessment within each experiment of whether any changes in engraftment could be explained by the contribution of the microenvironment to altered hematopoietic function. The probability of leukemia development or death over 16 weeks posttransplant was higher in the NHD13 recipients than in the WT recipients of NHD13 marrow (P = .01) (Figure 6D). These data show that the NHD13 BMME contributes to disease progression in NHD13 mice.
Hematopoiesis is improved in a WT BMME in transplantation models with low and intermediate MDS engraftment
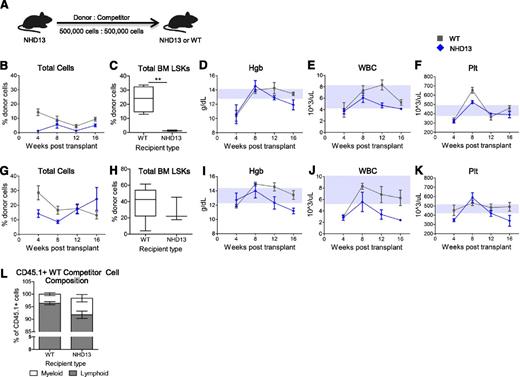
To directly test the impact of a WT vs NHD13 BMME on hematopoiesis during the MDS phase of this model, we analyzed blood and marrow of WT and NHD13 recipients of the same NHD13 marrow (Figure 7A). We noted that individual donors had very different degrees of engraftment in both WT and littermate NHD13 recipients. It was therefore necessary to examine donors individually. In NHD13 and WT recipients with low NHD13 donor engraftment in both WT and NHD13 recipients (Figure 7B-F), NHD13-derived cells engrafted better in the WT recipients than in the NHD13 recipients (Figure 7B and supplemental Figure 4A), suggesting that exposure of NHD13 marrow to the normal microenvironment improved hematopoietic function. NHD13-derived hematopoiesis was more myeloid skewed in NHD13 recipients than in WT recipients (supplemental Figure 4B), potentially indicating more NHD13 dysfunction in the context of an NHD13 BMME than in a WT BMME. Moreover, the contribution of NHD13-derived cells to the HSPC pool was significantly lower in NHD13 recipients as compared with WT recipients (Figure 7C), whereas total LSK frequency and marrow cellularity in the NHD13 and WT recipients was similar (0.07 ± 0.004 vs 0.12 ± 0.020% and 2.5 × 106 ± 7 × 105 vs 2.7 × 106 ± 8 × 104 cells, respectively). In this model, the total WBC counts were higher in the WT recipients relative to the NHD13 recipients (Figure 7E).
Hematopoiesis is improved when MDS marrow is exposed to a WT BMME. (A) Schematic of competitive repopulation assays in which NHD13 donor BM was transplanted with WT competitor BM into NHD13 recipients (blue diamonds) and WT recipients (gray squares). (B-F) Donor BM was from a 20-week-old NHD13 mouse transplanted into either lethally irradiated 23-week-old NHD13 mice (n = 3) or lethally irradiated WT littermates (n = 4). Comparison of engraftment of NHD13 marrow (B) in WT recipients vs NHD13 recipients (2-way ANOVA: P < .0001). At 16 weeks following transplant, whole BM of recipients was analyzed to determine contribution of NHD13 donor marrow to the total LSK pool (C) in WT and NHD13 recipients. Serial hematologic parameters hemoglobin (Hgb) (D), WBC count (E), and platelet count (Plt) (F) were measured in recipient mice every 4 weeks from time of transplant. (G-K) Donor BM was from a 15-week-old NHD13 mouse transplanted into either lethally irradiated 15-week-old NHD13 mice (n = 3) or WT littermates (n = 5). Comparison of engraftment of NHD13 marrow (G) in WT recipients vs NHD13 recipients (2-way ANOVA: P > .05). At 16 weeks following transplant, whole BM of recipients was analyzed to determine contribution of NHD13 donor marrow to the total LSK pool (H) in WT and NHD13 recipients. Serial hematologic parameters Hgb (I), WBC count (J), and Plt (K) were measured in recipient mice every 4 weeks from time of transplant. (L) Frequency of myeloid vs lymphoid cells within CD45.1+ WT competitor-derived cells in peripheral blood of all WT and NHD13 recipients at 16 weeks posttransplant. For hematologic data, upper and lower borders of gray boxes represent the interquartile range of Hgb, WBC, or Plt values for nontransplanted WT mice (from Figure 1).
Hematopoiesis is improved when MDS marrow is exposed to a WT BMME. (A) Schematic of competitive repopulation assays in which NHD13 donor BM was transplanted with WT competitor BM into NHD13 recipients (blue diamonds) and WT recipients (gray squares). (B-F) Donor BM was from a 20-week-old NHD13 mouse transplanted into either lethally irradiated 23-week-old NHD13 mice (n = 3) or lethally irradiated WT littermates (n = 4). Comparison of engraftment of NHD13 marrow (B) in WT recipients vs NHD13 recipients (2-way ANOVA: P < .0001). At 16 weeks following transplant, whole BM of recipients was analyzed to determine contribution of NHD13 donor marrow to the total LSK pool (C) in WT and NHD13 recipients. Serial hematologic parameters hemoglobin (Hgb) (D), WBC count (E), and platelet count (Plt) (F) were measured in recipient mice every 4 weeks from time of transplant. (G-K) Donor BM was from a 15-week-old NHD13 mouse transplanted into either lethally irradiated 15-week-old NHD13 mice (n = 3) or WT littermates (n = 5). Comparison of engraftment of NHD13 marrow (G) in WT recipients vs NHD13 recipients (2-way ANOVA: P > .05). At 16 weeks following transplant, whole BM of recipients was analyzed to determine contribution of NHD13 donor marrow to the total LSK pool (H) in WT and NHD13 recipients. Serial hematologic parameters Hgb (I), WBC count (J), and Plt (K) were measured in recipient mice every 4 weeks from time of transplant. (L) Frequency of myeloid vs lymphoid cells within CD45.1+ WT competitor-derived cells in peripheral blood of all WT and NHD13 recipients at 16 weeks posttransplant. For hematologic data, upper and lower borders of gray boxes represent the interquartile range of Hgb, WBC, or Plt values for nontransplanted WT mice (from Figure 1).
In a transplant model of MDS with an intermediate engraftment (Figure 7G-K), NHD13 marrow engraftment was initially improved in a WT compared with an NHD13 microenvironment (Figure 7G and supplemental Figure 4C). In spite of equal contribution of NHD13 marrow to the marrow LSK pool (Figure 7H), WT recipients had higher hemoglobin levels (Figure 7I) and higher WBC counts as compared with NHD13 recipients (Figure 7J). The superior hematologic parameters in the WT recipients therefore suggest that in this MDS model with intermediate engraftment of NHD13-derived donor cells, the MDS BMME is less supportive of hematopoiesis than a WT BMME.
In a model of MDS where there was high degree of NHD13 donor marrow engraftment (supplemental Figure 4D), NHD13 marrow engrafted more efficiently in NHD13 recipients compared with WT recipients. There were no differences in the measured hematologic parameters between the WT and the NHD13 recipients (supplemental Figure 4D). Together these data suggest that there is a threshold of NHD13 marrow engraftment above which normalization of the BMME and the associated improvement in support of WT hematopoiesis is not sufficient to improve overall hematopoiesis.
Interaction of WT marrow with an MDS BMME can induce an MDS phenotype
Our data thus far suggest that a WT BMME can improve hematopoietic function and delay death and/or development of leukemia in the setting of MDS. To determine if the MDS BMME can negatively impact WT hematopoietic cells, we analyzed WT competitor-derived CD45.1 cells at 16 weeks posttransplant in NHD13 and WT recipients of NHD13 marrow. Surprisingly, NHD13 recipients showed increased frequency of CD11b+ myeloid cells among CD45.1+ WT competitor-derived cells compared with WT recipients (Figure 7L). This suggests that the interaction of WT marrow with an MDS BMME can induce WT cells to develop an MDS phenotype of myeloid skewing.
Discussion
Although MDS is recognized as a spectrum of neoplastic myeloid disorders, the role of BMME in disease progression has long been postulated.31 Moreover, there is evidence from a murine model that a BMME defect can initiate MDS, suggesting that the BMME can also contribute to disease pathogenesis.32 Xenotransplantation experiments in mice have shown enhancement of engraftment of HSCs from patients with MDS when they were coinjected with BMME cells, indicating that these HSCs are dependent on signals from the BMME.33-35 In fact, in vitro evidence shows that exposure of BMME cells to hematopoietic cells from MDS patients can cause characteristic changes in the BMME cells, suggesting that hematopoietic MDS cells are able to modify the BMME.35 We used the NHD13 model to investigate the role of the BMME in MDS. The NHD13 model has important advantages that make it suitable for studying the BMME in the setting of MDS. First, the NHD13 mice have relatively long life spans, and therefore, exposure of the BMME to abnormal hematopoietic cells is presumably of a sufficient duration to be able to evaluate their impact on the BMME. Second, as opposed to xenograft models, the behavior and phenotypes of hematopoietic and microenvironmental cells are not confounded by the interactions from different species.
NHD13 mice were found to have depleted HSPC pools. The time-dependent decline in the MPP pool is most remarkable because this population was recently demonstrated to be critical for the daily regenerative needs of the hematopoietic system.24 It is likely that this decrease is in part because of primary changes in the HSCs induced by the transgene. However, based on the well-established impact of the BMME on HSC behavior, we hypothesized that in MDS the microenvironment contributes to the HSPC decline and dysfunction that we observed in the NHD13 transgenic mice.
Analysis of the BMME identifies several abnormalities that strongly suggest the microenvironment is modified by MDS-initiated signals. We detected increases in endothelial cell populations in the MDS compared with WT microenvironment. These data are particularly intriguing because recent data suggest that vascular niches regulate HSC activity.36 In fact, multiple reports identify endothelial dysfunction in patients with MDS.37-41 Therefore, although further studies are required to confirm that the three-dimensional architecture of this murine model approximates that found in human MDS, the NHD13 model is likely to faithfully replicate the BMME changes initiated by at least some subsets of human MDS. The clinical relevance of our murine model is also supported by the increase in VEGF, similar to what is reported in human MDS.42 We also noted a decrease in megakaryocytes in NHD13 mice, which may account for some of the HSC loss given the recently reported important role of megakaryocytes in HSC regulation.43-45
The increase in phenotypic but not functional MSCs may represent abnormalities in differentiation of these cell populations and needs to be further examined. In addition, in the MDS model there was an expansion in dysfunctional osteolineage cells, a phenotype analogous to the changes previously reported in an in vivo model of chronic myelogenous leukemia, suggesting that this may be a common feature of microenvironmental disruption by malignant hematopoietic pathologies.29 Notably, we and others have implicated the chemokine CCL3 in the osteoblastic dysfunction that is associated with a number of hematopoietic malignancies.29,30,46,47 CCL3 was increased in NHD13 mice, potentially providing mechanistic insight to the osteoblastic dysfunction. CCL3 has been reported to be increased in a subset of human MDS48 ; however, whether this is associated with decreased osteoblastic function is unknown.
The complexity of these microenvironmental changes confirms the importance of evaluating microenvironmental-hematopoietic interactions in vivo. To determine in vivo if hematopoietic function is improved by normalizing the microenvironment, we established a transplantation model where we could expose the same NHD13 marrow to either a WT or an NHD13 recipient microenvironment. This model demonstrated that improvement of the marrow microenvironment can ameliorate hematopoiesis, suggesting that targeting the microenvironment is a viable therapeutic option in MDS.
We identified myeloid shifting in NHD13-generated hematopoiesis, a reported characteristic of aging HSCs.27 In mice, altered Wnt signaling in HSCs has been shown to have a critical role in this alteration of phenotype with aging.49 Further experiments are needed to determine whether altered Wnt signaling in HSCs is also playing a role in myeloid shifting of NHD13 mice, and if so, whether it is mediated via interactions of HSCs with the BMME. This myeloid shift was recapitulated in our transplantation models of MDS and was mitigated by the normal microenvironment in mice with low NHD13 marrow engraftment. Further supporting the critical effect of the malignant microenvironment on hematopoietic dysfunction, we found significant myeloid shifts in competitor cells exposed to NHD13 but not WT recipients. Therefore, even genetically normal HSCs become dysfunctional when exposed to an MDS microenvironment.
We used marrow from NHD13 mice to develop a transplant model of MDS, which we consider to be analogous to human MDS in the sense that WT competitor marrow represents residual normal hematopoiesis while donor marrow represents myelodysplastic hematopoiesis. These models were set up as competitive transplants because of concern that the recipient mice of NHD13 marrow alone would die before we would have an opportunity to observe any differential BMME effects. Although we cannot exclude the possibility that the recipient marrow contributes to the total marrow HSPC pool, it is likely that the CD45.2-positive cells in both NHD13 and WT recipients originate from the donor mice, given that recipients received lethal doses of radiation prior to transplantation. Data from this transplant model strongly support the concept that the MDS BMME contributes to the MDS phenotype and to disease progression. Utilizing the NHD13 competitive transplant model, we showed that development of leukemia and death was significantly decreased in the presence of a WT BMME as compared with an MDS BMME, where the latter had a median overall survival that was as expected based on that of nontransplanted NHD13 mice. It is possible that some of the early mortality in our transplantation model may be because of marrow failure in the setting of recovery from radiation rather than leukemia; however, in both cases this increase in mortality is likely to be because of hematopoietic failure, which is mitigated by the normal microenvironment.
Based on our data from the NHD13 competitive transplant model, the role of the BMME in MDS may vary depending on the degree of myelodysplastic contribution in the marrow. When the MDS contribution is relatively low, the MDS BMME is less supportive of hematopoiesis compared with a WT BMME. In this scenario, there is evidence from our model that NHD13-derived hematopoietic dysfunction can be mitigated in the presence of a WT BMME. When MDS engraftment is high, the MDS BMME is more supportive of the MDS marrow compared with a WT BMME, and exposure to a WT BMME is not sufficient to improve cytopenias. This finding is consistent with the effect of leukemia on the BMME reported in xenograft models, where leukemic cells alter the recipient BMME, which becomes more supportive of leukemic HSCs than normal HSCs.50 In both the low and intermediate MDS engraftment models, the initial improvements in hematopoietic function in the presence of a WT BMME were transient; we speculate that there may be a progressive effect of the NHD13 marrow on the initially normal WT recipient microenvironment.
In conclusion, we used the NHD13 model to show, for the first time in vivo, direct evidence that the BMME has a key role in hematopoietic phenotype and progression, where exposure to a normal microenvironment can improve the performance of myelodysplastic hematopoietic cells. Further investigation is required to determine the mechanism by which the MDS BMME is altered and to discover which changes are most crucial with regard to HSPC support. However, our findings provide a strong rationale for a strategy where therapeutic manipulation of the BMME improves hematopoietic function and decreases transformation to leukemia in MDS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank John Ashton for providing technical assistance, Andrea Baran for guidance on statistical analyses, Elizabeth LaMere for maintaining mouse colonies, Dr Marshall Lichtman for critical reading of the manuscript, and all members of the Marrow Failure Research Group at the University of Rochester School of Medicine for helpful discussion.
This work was supported by the Wilmot Foundation (S.R.B.) and the National Institutes of Health, National Institute of Aging grant AG046293 (L.M.C. and M.W.B.), National Cancer Institute grant CA166280 (L.M.C. and M.W.B.), and National Institute of Diabetes and Digestive and Kidney Diseases grant DK081843 (L.M.C.). This work was also supported by a charitable donation from Frank and Barbara Strong.
Authorship
Contribution: S.R.B., L.M.C., M.W.B., B.J.F., and A.J.L. designed experiments; S.R.B., B.J.F., A.J.L., A.N.G., M.W.L., M.A.G., and C.M.H. performed experiments; S.R.B., B.J.F., M.A.G., L.M.C., M.W.B., and A.J.L. performed analyses of results; S.R.B., L.M.C., M.W.B., B.J.F., A.J.L., J.L.L., and A.G.E. interpreted experimental results; and S.R.B., A.J.L., and L.M.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laura M. Calvi, Endocrine Division, Department of Medicine, University of Rochester School of Medicine, 601 Elmwood Ave, Box 693, Rochester, NY 14642; e-mail: laura_calvi@urmc.rochester.edu.
References
Author notes
S.R.B. and A.J.L. contributed equally to this study.

![Figure 1. NHD13 mice develop cytopenias, macrocytosis, and myeloid dysplasia, with increased rate of progression to leukemia or death. (A-D) Male and female NHD13 (blue diamond) and wild-type (WT; gray square) littermates were followed with serial complete blood count measurements (WT, n = 2-12; NHD13, n = 3-14). Data from leukemic animals censored at the first time point at which they were noted to be leukemic. Results analyzed for statistical significance using 2-way analysis of variance (ANOVA). P = .0025 for granulocytes; P < .0001 for all other parameters. (E) Normal neutrophil in the peripheral blood of a WT mouse. (×1000 oil immersion). (F) Dysplastic neutrophil in the peripheral blood of representative NHD13 mouse (×1000 oil immersion). (G) Flow cytometric analysis of mononuclear cells in peripheral blood (myeloid, CD11b+; lymphoid, CD3e+ or B220+). (H) Survival curves showing progression to leukemia or death in NHD13 or WT mice (log-rank [Mantel-Cox] test: P = .09). (I) Schematic of transplant setup. Spleen cells (1 x 106) from NHD13 mouse (CD45.2) noted to have high peripheral WBC counts and massive splenomegaly were transplanted to WT CD45.1 recipients. (J) Leukemic donor cells demonstrated engraftment of >90% in recipients at 3 weeks posttransplant. (K) Representative spleens from nontransplanted WT (left) and recipients of NHD13 cells (right) demonstrating splenomegaly consistent with leukemic development at 3 weeks posttransplant. (L) Representative peripheral blood smears from recipients showing blasts consistent with leukemic development at 3 weeks posttransplant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/5/10.1182_blood-2015-06-653113/4/m_616f1.jpeg?Expires=1765884446&Signature=sZbprA9AIABtayDmpiDNsoZIm9x-wNTSPZkzUtMgOeu2c9UgaMbuV~Ivnnft8ykbFwQbuhjqGQjiKp8z90LmlHUDemCuTQlaFLhZAI7MfEs-DZMUBJc0pp6ol1qOxzqXKlii3RcmtEyrl5ClStJpdr1lnnQ8rQbtfDpck50bWHg2ACxB7PiQaPaF~XUHVQ40AzY5dgO-7wS556xYu2GIl4~VdE2d3btSypIu9sV1jzfdJ6H3kFytQ-t8a3Ov38K7NSVFKZTtvD02iU7oQMGJY4MaN0LUjLkHf-t3ejw~2J4rA44324qoS1V6EXtmJFisbrMH1BXzY~pQgT3husr7Ug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal