Key Points
Function and maturation of myeloid DCs is abnormal in AP-3 complex-deficient patients.
IFN-α secretion in response to HSV-1 is reduced in AP-3–deficient patients.
Abstract
Hermansky-Pudlak syndrome type 2 (HPS2) is a primary immunodeficiency due to adaptor protein-3 (AP-3) complex deficiency. HPS2 patients present neutropenia, partial albinism, and impaired lysosomal vesicles formation in hematopoietic cells. Given the role of dendritic cells (DCs) in the immune response, we studied monocyte-derived DCs (moDCs) and plasmacytoid DCs (pDCs) in two HPS2 siblings. Mature HPS2 moDCs showed impaired expression of CD83 and DC–lysosome-associated membrane protein (LAMP), low levels of MIP1-β/CCL4, MIG/CXCL9, and severe defect of interleukin-12 (IL-12) secretion. DCs in lymph-node biopsies from the same patients showed a diffuse cytoplasm reactivity in a large fraction of DC-LAMP+ cells, instead of the classical dot-like stain. In addition, analysis of pDC-related functions of blood-circulating mononuclear cells revealed reduced interferon-α secretion in response to herpes simplex virus-1 (HSV-1), whereas granzyme-B induction upon IL-3/IL-10 stimulation was normal. Finally, T-cell costimulatory activity, as measured by mixed lymphocyte reaction assay, was lower in patients, suggesting that function and maturation of DCs is abnormal in patients with HPS2.
Introduction
Hermansky-Pudlak syndrome type 2 (HPS2) is an autosomal recessive disease caused by mutations of the AP3B1 gene, encoding for the beta3A subunit of the heterotetrameric adaptor protein-3 complex. The adaptor protein-3 (AP-3) complex is involved in protein sorting and in cargo selective transport of proteins from endosomes to the lysosome. All HPS2 patients described to date are homozygous or compound heterozygous for mutations in the AP3B1 gene, resulting in the complete absence of beta3A expression.1-4
HPS2 patients display recurrent viral and bacterial infections due to defects of cytotoxic T cells, natural killer cells,5,6 and CD1-dependent antigen presentation.7,8 Neutrophils are also involved, but neutropenia that is observed in all HPS2 patients is usually responsive to granulocyte colony-stimulating factor (CSF) treatment, suggesting that the neutrophil abnormalities do not account for the susceptibility to infections.9
There is evidence that AP-3 is involved in toll-like receptor (TLR) signaling and major histocompatibility complex functions of murine dendritic cells (DCs).10 In addition, AP-3 is essential for TLR7- and TLR9-dependent secretion of type I interferon (IFN) by plasmacytoid DCs (pDCs).11,12 These studies suggest that DCs might play a role in the pathogenesis of infections in HPS2.
Therefore, we studied the maturation of monocyte-derived DCs (moDCs) in two siblings with HPS213 by analysis of CD83 and DC–lysosome-associated membrane protein (DC-LAMP) expression, and cytokine secretion. We also observed a low percentage of circulating myeloid DCs (mDCs) and pDCs. Moreover, following in vitro stimulation, peripheral blood mononuclear cells (PBMCs) were unable to secrete IFN-α upon herpes simplex virus-1 (HSV-1) infection. Finally, analysis of the costimulatory activity of mDCs for lymphocytes showed that DCs from AP-3–deficient patients display delayed maturation and abnormal cytokine secretion.
Study design
Monocytes were differentiated into DCs by the addition of granulocyte-macrophage CSF (GM-CSF) 50 ng/mL and 20 ng/mL interleukin-4 (IL-4) (PeproTech). After 6 days of culture, analysis of human leukocyte antigen–antigen D related (HLA-DR) showed that at least 80% of cells were CD1a+/CD14−. Final maturation was induced by stimulation with 100 ng/mL lipopolysaccharide (LPS) (Sigma-Aldrich), 500 U/mL IFN-γ (R&D Systems), or with multimeric soluble human CD40 ligand at 1 μg/mL (CD40L; Miltenyi Biotec, Bergisch Gladbach, Germany) for an additional 24 or 48 hours.
In immunofluorescence studies, cells were seeded on glass slides by cytospin and permeabilized with 100% ice-cold methanol. Immunohistochemistry (IHC) studies were performed on formalin-fixed paraffin-embedded sections obtained from nodular lymphocyte predominant Hodgkin lymphoma biopsies.
More details of experimental procedures, materials, and analysis are provided in supplemental Materials and Methods, available on the Blood Web site.
Results and discussion
Impaired maturation of moDCs in HPS2 patients
In order to study DCs in HPS2 patients, we cultured adherence-purified monocytes with IL-4 and GM-CSF, and added LPS to cultures to induce full maturation of DCs. We evaluated moDCs maturation by immunostaining with anti–DC-LAMP, anti–HLA-DR, and anti-CD83 moAbs. After 24 hours stimulation, DC-LAMP expression by HPS2 moDCs was lower in comparison with cells derived from healthy subjects (Figure 1A). Likewise, the analysis of CD83 expression in DCs of HPS2 patients revealed a striking difference of CD83 staining, suggesting that DCs from HPS2 patients have a defect of cell maturation (Figure 1A). However, after 48 hours of culture, DC-LAMP became detectable (supplemental Figure 1). CD83 expression was detectable with a 0.9- and 1.4-fold increase, respectively, in the 2 HPS2 patients as compared with a fivefold upregulation in control moDCs. DC activation by the use of soluble multimeric CD40-ligand resulted in a 1.6- and 2.2-fold increase of CD83 in the 2 HPS2 patients as compared with a 5.5-fold upregulation in control moDCs. These results suggest that DCs in HPS2 patients have a delay in DC activation rather than a complete defect in maturation.
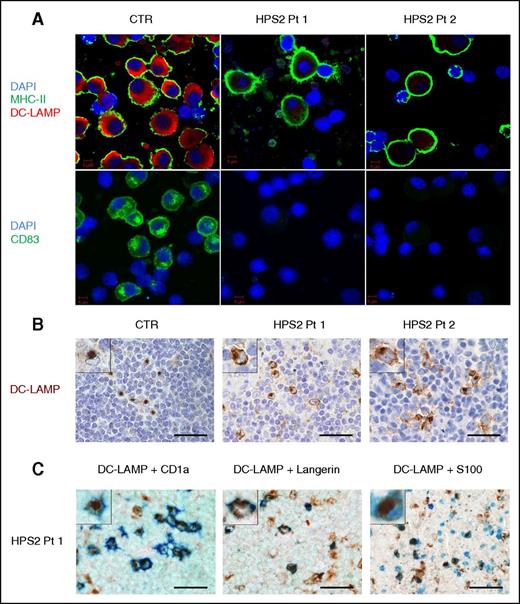
Altered expression of maturation markers in HPS2 DCs. (A) moDCs from HPS2 patients and control were induced to mature by culture with LPS for 24 hours, seeded on glass, and permeabilized with methanol. DCs were stained with moAbs against DC-LAMP (red) or HLA-DR (green) (top panels), and with anti-CD83 (green) and DAPI (blue) (bottom panels). Images were acquired using a confocal microscope (LSM 510 META; Carl Zeiss Inc.) with a ×100/1.3 oil objective. Images were acquired sequentially using separate laser excitations to avoid crosstalk between the fluorophore signals. (B-C) IHC on formalin-fixed paraffin-embedded sections obtained from lymph nodes of Pt1, Pt2, and control. In the lymph nodes from HPS2-patients, DC-LAMP reactivity is diffused in the cytoplasm, whereas in control, DC-LAMP reactivity is limited to the classical paranuclear dot (B, inserts). By double IHC (C), DC-LAMP+ cells (brown) coexpress CD1a (41% of DC-LAMP+ cells), Langerin (45%), and S100 protein (62%) (blue). CTR, control; DAPI, 4′,6-diamidino-2-phenylindole; MHC, major histocompatibility complex; Pt, patient.
Altered expression of maturation markers in HPS2 DCs. (A) moDCs from HPS2 patients and control were induced to mature by culture with LPS for 24 hours, seeded on glass, and permeabilized with methanol. DCs were stained with moAbs against DC-LAMP (red) or HLA-DR (green) (top panels), and with anti-CD83 (green) and DAPI (blue) (bottom panels). Images were acquired using a confocal microscope (LSM 510 META; Carl Zeiss Inc.) with a ×100/1.3 oil objective. Images were acquired sequentially using separate laser excitations to avoid crosstalk between the fluorophore signals. (B-C) IHC on formalin-fixed paraffin-embedded sections obtained from lymph nodes of Pt1, Pt2, and control. In the lymph nodes from HPS2-patients, DC-LAMP reactivity is diffused in the cytoplasm, whereas in control, DC-LAMP reactivity is limited to the classical paranuclear dot (B, inserts). By double IHC (C), DC-LAMP+ cells (brown) coexpress CD1a (41% of DC-LAMP+ cells), Langerin (45%), and S100 protein (62%) (blue). CTR, control; DAPI, 4′,6-diamidino-2-phenylindole; MHC, major histocompatibility complex; Pt, patient.
Because both patients underwent lymph node biopsy for lymphoproliferative disease, we explored DC-LAMP expression in the context of HPS2-associated Hodgkin lymphoma. We could detect DC-LAMP+ cells in the extranodular non-neoplastic T-cell area of both patients and controls (Figure 1B). Remarkably, the subcellular distribution of DC-LAMP in patients’ tissue was abnormal in a large fraction of positive cells, consisting of a diffuse reactivity in the cytoplasm instead of the classical dot-like, paranuclear Golgi stain (Figure 1B, insert). By double IHC, numerous DC-LAMP+ cells coexpressed S100 (62%), Langerin (45%), and CD1a (41%) (Figure 1C) with no immunoreactivity with anti-CD123, -CD163, and -CD14 moAbs (not shown), thus suggesting a Langerhans-derived interdigitating DC identity for many of them.
Altered cytokine production by mature moDCs and pDCs of HPS2 patients
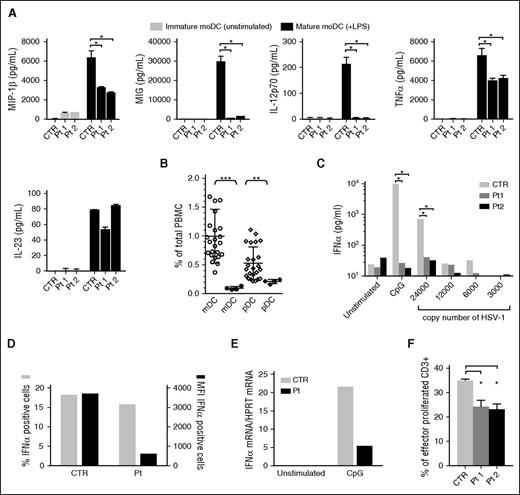
Because DCs are the main producers of cytokines that regulate T-cell activation, we decided to evaluate chemokine and cytokine production by moDCs after culture for 24 hours with LPS. Stimulated DCs showed a defective production of MIP1-β/CCL4 and MIG/CXCL9, two chemokines that induce migration of activated T cells, normal secretion of IL-23, reduced TNF-α levels, and a complete defect in IL-12 production (Figure 2A). This result is consistent with those obtained in pearl mice.10 Given the defect of IL-12 production by HPS2 moDCs, it is likely that T cells of HPS2 patients might display impaired T helper 1 polarization.10 Because IFN-γ has the unique capacity to prime DCs for high IL-12 production, we investigated the effect of this cytokine on IL-12 secretion.14,15 Results reported in supplemental Figure 2A show that the combined stimulation with LPS and IFN-γ was able to restore IL-12 secretion to normal levels, suggesting that under optimal conditions of stimulation, IL-12 production is restored. In addition, the secretion by mature moDCs of other cytokines and chemokines, such as MIP1-α/CCL3, granulocyte-CSF, and IL-6 was normal, except for an evident overproduction of MCP-1/CCL2 and IL-8/CXCL8 by HPS2 immature DCs (supplemental Figure 2B). Higher production of MCP-1/CCL2 and IL-8/CXCL8 by HPS2 DCs in the absence of stimulation might reflect the constitutive activation of pro-inflammatory pathways in these cells. The abnormal pattern of IL-8/CXCL8 secretion may contribute to the altered neutrophil homeostasis observed in HPS2 patients.
Impairment of DC functions in HPS2 patients. DCs were generated from freshly isolated monocytes by adding IL-4 and GM-CSF for 6 days. Final maturation has been induced after the addition of LPS for another 24 hours. Analysis of MIP1-β/CCL4, MIG/CXCL9, IL-12, TNF-α, and IL-23 production (A, all panels) by immature DCs (gray bars) and mature DCs (black bars) derived from HPS2 patients or from a healthy subject. Statistical analysis by non-parametric test shows a significant difference (*P < .05). (B) mDCs (○) and pDCs (♢) were evaluated by FACS analysis in peripheral blood from the HPS2 patients (● and ♦) as compared with the percentages detected in healthy subjects of comparable age (○ and ♢). Statistical analysis of DC counts shows that mDCs and pDCs were significantly lower in HPS2 patients after 2 independent measurements as compared with cell counts in 24 normal control subjects (**P < .005; ***P < .001). (C) IFN-α production, measured in triplicate, by freshly isolated PBMCs after stimulation for 24 hours with 6 μg/mL CpG, HSV-1 (copies/200 μL), or medium alone in HPS2 and 1 control subject. Statistical analysis by non-parametric test shows a significant difference (*P < .05). This experiment is representative of 2 experiments performed. (D) PBMCs were stimulated with 6 μg/mL CpG for 5 hours in the presence of brefeldin A for the last 3 hours. At the end of stimulation, PBMCs were stained with anti–BDCA2-FITC Ab and anti–CD123-Vioblue Ab to identify pDCs, and with an anti-IFNα–antigen-presenting cell Ab. The graph shows the percentage (gray bars) and (MFI, black bars) of IFN-α–positive cells. (E) PBMCs were stimulated with 6 μg/mL CpG for 5 hours. At the end of incubation, PBMCs were collected and IFN-α levels were evaluated by real-time polymerase chain reaction. Levels of HPRT messenger RNA were used for normalization. (F) MLR was performed by evaluation of the percentage of proliferating CD3+ cells after incubation with irradiated PBMCs from HPS2 patients or a control subject for 7 days. Statistical analysis by non-parametric test shows a significant difference (*P < .05). Ab, antibody; CTR, control; HPRT, hypoxanthine guanine phosphoribosyltransferase; MFI, mean fluorescence intensity; Pt, patient.
Impairment of DC functions in HPS2 patients. DCs were generated from freshly isolated monocytes by adding IL-4 and GM-CSF for 6 days. Final maturation has been induced after the addition of LPS for another 24 hours. Analysis of MIP1-β/CCL4, MIG/CXCL9, IL-12, TNF-α, and IL-23 production (A, all panels) by immature DCs (gray bars) and mature DCs (black bars) derived from HPS2 patients or from a healthy subject. Statistical analysis by non-parametric test shows a significant difference (*P < .05). (B) mDCs (○) and pDCs (♢) were evaluated by FACS analysis in peripheral blood from the HPS2 patients (● and ♦) as compared with the percentages detected in healthy subjects of comparable age (○ and ♢). Statistical analysis of DC counts shows that mDCs and pDCs were significantly lower in HPS2 patients after 2 independent measurements as compared with cell counts in 24 normal control subjects (**P < .005; ***P < .001). (C) IFN-α production, measured in triplicate, by freshly isolated PBMCs after stimulation for 24 hours with 6 μg/mL CpG, HSV-1 (copies/200 μL), or medium alone in HPS2 and 1 control subject. Statistical analysis by non-parametric test shows a significant difference (*P < .05). This experiment is representative of 2 experiments performed. (D) PBMCs were stimulated with 6 μg/mL CpG for 5 hours in the presence of brefeldin A for the last 3 hours. At the end of stimulation, PBMCs were stained with anti–BDCA2-FITC Ab and anti–CD123-Vioblue Ab to identify pDCs, and with an anti-IFNα–antigen-presenting cell Ab. The graph shows the percentage (gray bars) and (MFI, black bars) of IFN-α–positive cells. (E) PBMCs were stimulated with 6 μg/mL CpG for 5 hours. At the end of incubation, PBMCs were collected and IFN-α levels were evaluated by real-time polymerase chain reaction. Levels of HPRT messenger RNA were used for normalization. (F) MLR was performed by evaluation of the percentage of proliferating CD3+ cells after incubation with irradiated PBMCs from HPS2 patients or a control subject for 7 days. Statistical analysis by non-parametric test shows a significant difference (*P < .05). Ab, antibody; CTR, control; HPRT, hypoxanthine guanine phosphoribosyltransferase; MFI, mean fluorescence intensity; Pt, patient.
Flow cytometric analysis revealed a decreased percentage of circulating mDCs and pDCs, suggesting an abnormal trafficking of DCs from draining tissues to lymphoid organs and blood circulation (Figure 2B). Interestingly, analysis of blood cell counts in pearl mice has shown that the number of conventional DC and pDC is normal.16 Likewise, pearl mice are not neutropenic and do not have cyclic neutropenia, two events observed in HPS2 patients and canine cyclic hematopoiesis in dogs, respectively.17 Altogether, these observations highlight differences between HSP2 patients and the pearl mouse model. pDCs functions in AP-3–deficient patients were investigated in terms of IFN-α secretion in response to HSV-1 and cytosine guanine dinucleotide (CpG) stimulation (a TLR9 agonist). Because pDCs are the main IFN-α producers among hematopoietic cells,12,18 freshly isolated PBMCs were stimulated with serial dilutions of vital HSV-1 or CpG, and IFN-α levels evaluated after 24 hours. Surprisingly, supernatants from PBMCs obtained from HPS2 patients showed a complete lack of IFN-α production in comparison with the abundant levels detected with control cells (Figure 2C). To further investigate the impairment of IFN-α production in HPS2 pDCs, the expression of IFN-α was investigated following stimulation with CpG. Analysis of IFN-α protein production by intracellular fluorescence-activated cell sorter (FACS) analysis revealed a comparable number of IFN-α–producing cells in cells from HPS2 patients and control subjects. However, the strong reduction in the mean fluorescence intensity indicated that the degree of IFN-α production was strongly reduced in HPS2 patients (Figure 2D). The defective production of IFN-α in HPS2 patients was also confirmed at the messenger RNA level by real-time polymerase chain reaction (Figure 2E). Taking together, these results suggest that both the reduced number of circulating pDCs and the reduced ability to release IFN-α may concur to determine the impairment of IFN-α secretion by PBMCs from HPS2 patients. In contrast, granzyme B production upon IL-3/IL-10 stimulation, evaluated both in the cell supernatants and by intracellular staining, was normal (supplemental Figure 3). These results suggest that the AP-3 complex may play a specific, nonredundant role in TLR7 and TLR9 activation pathways of pDCs. This is in accord with previous observations11,12 and with the finding that pDCs from pearl mice fail to secrete type I IFN after murine cytomegalovirus infection in vitro.16
DCs of HPS2 patients display a reduced ability to induce T-cell proliferation
The antigen-presenting functions of DCs was investigated by analyzing the ability of freshly isolated irradiated PBMCs to induce proliferation of allogeneic normal T cells in the mixed lymphocyte reaction (MLR) assay.19,20 Analysis of proliferation of the responder T cells was evaluated by carboxyfluorescein diacetate succinimidyl ester staining. A significant proliferative response of T cells was observed with cells obtained from both HPS2 patients and the control subjects. However, the percentage of T cells that have entered the cell cycle after 7 days of culture was significantly lower when PBMCs from HPS2 patients were used to induce the T-cell response (Figure 2F; supplemental Figure 4). At the moment, it is unclear whether HPS2 DCs have a defective ability to induce T-cell proliferation or whether the reduced number of DCs present in the PBMCs obtained from HPS2 patients might be responsible for the reduced T-cell proliferation observed in the MLR assay.
Taken together, these observations suggest that AP-3 deficiency results in abnormal maturation of monocyte-derived DCs and impaired total antigen-presenting cell activity, as measured by MLR assay. Overall, we propose that the impairment of DC activity in HPS2 patients contributes to susceptibility to bacterial and viral infections observed in these patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marco Cassatella and his group, and in particular, Nicola Tamassia for IFN-α analysis.
This work was supported by grants from the European Union (FP7 HLH-cure, project #201461) and Progetti di Rilevante Interesse Nazionale (PRIN) 2009 (R.B.).
Authorship
Contribution: A. Prandini and V.S. codesigned and reviewed the study, and performed cellular studies and FACS analysis; F.C. set up immunocytology studies; D.M. performed FACS analysis; M.A.D.F. provided the herpes virus and performed viral studies; A. Plebani, L.D.N., and F.P. obtained clinical information; W.V., L.L., and F.F. performed immune-histopathology studies; S.S. made substantial contributions to interpretation of the data; and R.B. supervised the project and helped to write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raffaele Badolato, Institute of Molecular Medicine “Angelo Nocivelli,” University of Brescia, c/o Spedali Civili, 25123 Brescia, Italy; e-mail: raffaele.badolato@unibs.it.
References
Author notes
A.P. and V.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal