Key Points
MRD monitoring is one of the most relevant prognostic factors in elderly MM patients, irrespective of age or cytogenetic risk.
Second-generation MFC immune profiling concomitant to MRD monitoring also helped to identify patients with different outcomes.
Abstract
The value of minimal residual disease (MRD) in multiple myeloma (MM) has been more frequently investigated in transplant-eligible patients than in elderly patients. Because an optimal balance between treatment efficacy and toxicity is of utmost importance in patients with elderly MM, sensitive MRD monitoring might be particularly valuable in this patient population. Here, we used second-generation 8-color multiparameter-flow cytometry (MFC) to monitor MRD in 162 transplant-ineligible MM patients enrolled in the PETHEMA/GEM2010MAS65 study. The transition from first- to second-generation MFC resulted in increased sensitivity and allowed us to identify 3 patient groups according to MRD levels: MRD negative (<10−5; n = 54, 34%), MRD positive (between <10−4 and ≥10−5; n = 20, 12%), and MRD positive (≥10−4; n = 88, 54%). MRD status was an independent prognostic factor for time to progression (TTP) (hazard ratio [HR], 2.7; P = .007) and overall survival (OS) (HR, 3.1; P = .04), with significant benefit for MRD-negative patients (median TTP not reached, 70% OS at 3 years), and similar poorer outcomes for cases with MRD levels between <10−4 and ≥10−5 vs ≥10−4 (both with a median TTP of 15 months; 63% and 55% OS at 3 years, respectively). Furthermore, MRD negativity significantly improved TTP of patients >75 years (HR, 4.8; P < .001), as well as those with high-risk cytogenetics (HR, 12.6; P = .01). Using second-generation MFC, immune profiling concomitant to MRD monitoring also contributed to identify patients with poor, intermediate, and favorable outcomes (25%, 61%, and 100% OS at 3 years, respectively; P = .01), the later patients being characterized by an increased compartment of mature B cells. Our results show that similarly to transplant candidates, MRD monitoring is one of the most relevant prognostic factors in elderly MM patients, irrespectively of age or cytogenetic risk. This trial was registered at www.clinicaltrials.gov as #NCT01237249.
Introduction
We are witnessing a remarkable progress in multiple myeloma (MM), with several new drugs being recently approved1-5 and an armamentarium of emerging new agents with novel mechanisms of action showing promising efficacy,6 altogether resulting in a significant prolongation of patients’ survival.7 The increasing availability of drugs with well-balanced efficacy and toxicity profiles has led to the design of more complex and prolonged treatment strategies,8,9 but it has also raised the unmet need for surrogate markers to predict overall survival (OS) and accelerate the approval of new agents.10 Thus, there is a growing body of evidence indicating that minimal residual disease (MRD) assessment can potentially be used as a biomarker to evaluate the efficacy of different treatment strategies and potentially act as surrogate for OS, particularly among transplant-eligible patients11,12 ; however, it is perhaps in elderly MM patients, the most common subgroup and for which an optimal balance between efficacy and toxicity is of utmost importance, that sensitive response assessment could help to avoid under- or overtreatment. Unfortunately, the value of MRD monitoring in patients with elderly MM has only been investigated in 2 series of well-defined transplant-ineligible patients: the PETHEMA/GEM2005MAS65 study13 and the nonintensive pathway of the MRC Myeloma IX clinical trial.14 Although the achievement of MRD negativity predicted for a significant prolongation in time-to progression (TTP)15-17 and OS18 in the PETHEMA/GEM2005MAS65 study, no statistically significant differences in survival were noted between MRD-negative and positive patients in the nonintensive pathway of the MRC Myeloma IX clinical trial19 ; therefore, although recent studies indicate that high MRD-negative rates can be achieved by a significant number of transplant-ineligible patients treated with optimized therapeutic combinations,20,21 the clinical significance of MRD monitoring in patients with elderly MM remains an (important) open question.
Here, we investigated the role of MRD assessment in transplant-ineligible MM patients enrolled in the PETHEMA/GEM2010MAS65 clinical trial using a second-generation 8-color multiparameter flow cytometry (MFC) assay. Upon demonstrating the increased specificity and sensitivity of second- vs first-generation MFC, and the clinical relevance of MRD detection at 10−5 levels, we show that MRD negativity in elderly MM patients predicts prolonged survival, irrespective of a patient’s cytogenetics or age. Moreover, by taking further advantage of the second-generation 8-color MFC assay, we also showed for the first time that immune profiling of MM during MRD monitoring after therapy is prognostically relevant and allows the identification of patients with either poor survival or sustained disease control despite persistent MRD.
Patients and methods
Study design
The PETHEMA/GEM2010MAS65 is an open-label, phase 2 trial for newly diagnosed elderly MM patients randomized (1:1) into a sequential scheme consisting of 9 cycles of bortezomib, melphalan, and prednisone (VMP) followed by 9 cycles of lenalidomide and low-dose dexamethasone (Rd) or the same regimens in an alternating scheme (1 cycle of VMP alternating with 1 Rd, for up to 18 cycles).22 All samples were collected after informed consent was given by each patient, according to the local ethical committees and the Declaration of Helsinki. This trial was registered at www.clinicaltrials.gov as #NCT01237249.
Patients
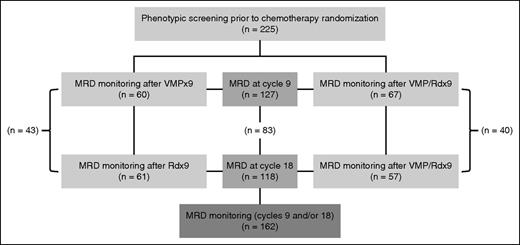
Overall, 162 out of 241 patients enrolled in the PETHEMA/GEM2010MAS65 had bone marrow (BM) aspirates monitored for MRD. Patient selection for MRD testing was based on the presence of M-component response; accordingly, 80% of the patients with BM aspirates centralized for MRD assessment were in very good partial response or better, and 50% were in complete remission as defined by the International Myeloma Working Group response criteria.23 The distribution of patients between treatment arms was well balanced (n = 78 and n = 84 for the sequential and alternating arms, respectively) (Figure 1). Median follow-up after enrollment of the 162 patients under study was of 36 months (and 30 months in the whole series of 241 patients22 ). At 36 months, 79 out of 162 patients (49%) had progressed and 34 out of 162 patients (21%) had died.
PETHEMA/GEM2010MAS65 MRD study consort diagram. A total of 225 patients were immunophenotyped at diagnosis, and 6 patients (3%) were excluded from further MRD monitoring due to the lack of aberrant phenotypes. A total of 127 patients had MRD assessed at cycle 9 after consecutive cycles of VMP (n = 60) or alternating VMP/Rd (n = 67). A total of 118 patients had MRD assessed at cycle 18 after sequential VMP followed by Rd (n = 61) or alternating VMP/Rd (n = 57), 83 of them with MRD data on cycles 9 and 18. Thus, 162 patients enrolled in the PETHEMA/GEM2010MAS65 had at least 1 MRD study.
PETHEMA/GEM2010MAS65 MRD study consort diagram. A total of 225 patients were immunophenotyped at diagnosis, and 6 patients (3%) were excluded from further MRD monitoring due to the lack of aberrant phenotypes. A total of 127 patients had MRD assessed at cycle 9 after consecutive cycles of VMP (n = 60) or alternating VMP/Rd (n = 67). A total of 118 patients had MRD assessed at cycle 18 after sequential VMP followed by Rd (n = 61) or alternating VMP/Rd (n = 57), 83 of them with MRD data on cycles 9 and 18. Thus, 162 patients enrolled in the PETHEMA/GEM2010MAS65 had at least 1 MRD study.
Second-generation MFC
A single 8-color antibody combination (CD45-PacB, CD138-OC515, CD38-FITC, CD56-PE, CD27-PerCPCy5.5, CD19-PECy7, CD117-APC, and CD81-APCH7) was used to discriminate between phenotypically aberrant and normal plasma cells (PCs), and MRD negativity was defined when <20 clonal PCs were detected among ≥2 × 106 leukocytes (<0.001%; limit of detection, 10−5). Briefly, phenotypically aberrant PCs were identified according to underexpression of CD19, CD27, CD38, CD45, and/or CD81; overexpression of CD56; and asynchronous expression of CD117. A minimum of 2 aberrant phenotypes (eg, coexpression of CD56 and CD117) were required to define a cluster of clonal PCs. Six of the 225 patients (3%) had, according to their diagnostic immunophenotyping (following EuroFlow guidelines24 ) during enrollment in the PETHEMA/GEM2010MAS65 study, light-chain restricted clonal PCs lacking aberrant phenotypes for all markers tested. Because light chains were not assessed with the second-generation MFC assay, MFC-based MRD monitoring was considered not applicable for these 6 cases; therefore, the applicability of the second-generation MFC assay was 97%. The 8-color combination also allowed for the enumeration of erythroid (CD117+, CD38−/dim, CD45−/dim, SSClo) and myeloid (CD117+, CD38+, CD45dim, SSChi) hematopoietic progenitors, erythroblasts (CD45−, CD38−, SSClo), mast cells (CD117bright, CD45dim), eosinophils (CD45bright, CD81bright, SSChi), basophils (CD38+, CD81−, CD45dim), monocytes (CD45+, CD38+, CD81+, SSCint), neutrophils (CD45dim, CD81−, SSChi), B lymphocytes and their respective precursor (CD19+, CD45dim, CD38bright, CD27−), naïve (CD19+, CD45+, CD38−/dim, CD27−), and memory (CD19+, CD45+, CD38−/dim, CD27+) subsets, as well as natural killer T cells plus natural killer cells (CD45+, CD56+, CD19−, SSClo) and remaining T lymphocytes (CD45+, CD56−, CD19−, SSClo); such data were used to generate individual immune profiles for 146 patients. Briefly, principal component analysis (PCA) based on the 13 cell subsets enumerated was performed using the automated population separator (principal component 1 vs principal component 2) graphical representation and multivariate analysis tool of the Infinicyt software (Cytognos SL, Salamanca, Spain), as described elsewhere.25 BM samples were acquired in a FACSCantoII flow cytometer using the FACSDiva software program (Becton Dickinson Bioscience, San Jose, CA), and data were analyzed with the Infinicyt software. MRD assessment was centralized in three PETHEMA/GEM laboratory cores, cytometrists were blinded to all clinical data, and results were prospectively uploaded into a locked intranet dataset.
Cytogenetic characterization
Interphase fluorescence-in-situ-hybridization (FISH) was performed at diagnosis on immunomagnetic-enriched PCs from 132 out of 162 cases with MRD assessment after therapy. Patients were tested for IGH translocations, +1q, and del(17p13); those cases displaying a t(4;14), t(14;16), and/or del(17p13) were classified as having high-risk disease (n = 26) and all other cases as standard risk (n = 106).
Statistical analyses
The Kruskal-Wallis test was used to estimate the statistical significance of differences observed between groups. Survival curves were plotted by the Kaplan-Meier method and compared by the 2-sided log-rank test. TTP was defined as the time from MRD assessment to disease progression and OS as time from MRD assessment to death from any cause. A multivariate Cox proportional hazard model was developed to explore the independent value of variables with significant impact on the univariate analysis, and variables were retained in the model for levels of significance P < .05. The SPSS software (version 20.0; IBM, Chicago, IL) was used for all statistical analyses.
Results
Second-generation MFC-based MRD monitoring
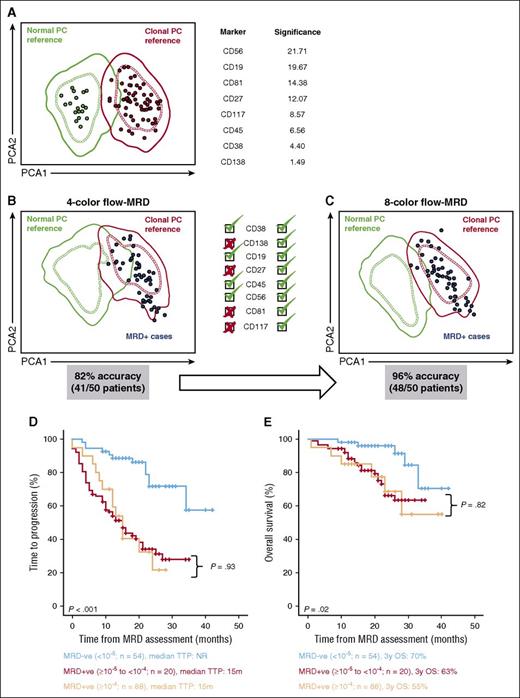
In a first step, we determined the differences in specificity and sensitivity between the second-generation 8-color MFC assay and the first-generation test, based in only 4-markers (CD19, CD38, CD45 and CD56) and the evaluation of 2 × 105 cells. For this purpose, we created a reference database consisting of normal and clonal PCs in order to determine, by PCA, the individual contribution of the novel markers to discriminate between both PC populations (Figure 2A). CD56 ranked as the most significant marker followed by CD19, CD81, CD27, CD117, CD45, CD38, and CD138; thus, up to 3 new markers (ie, CD81, CD27, and CD117) ranked higher than CD45 and CD38. Afterward, we focused on 50 randomly selected MRD-positive patients enrolled in this study to compare, according to the reference database, the performance of 4- vs 8-color discrimination between clonal and normal PCs. PCA of 4-color data showed MRD cells from 9 out of 50 patients to be located in the overlapping area between 1 and 2 standard deviations (SDs) of the normal and clonal PC references (82% accuracy; Figure 2B); by contrast, in PCA of 8-color data, all but 2 patients were accurately located in the clonal PC reference, outside 1 or 2 SD curves of the normal PC reference (96% accuracy; Figure 2C). To investigate the potential increment in sensitivity introduced by second-vs first-generation MFC, we used the Infinicyt software to reduce the total number of analyzed cells from 2 × 106 (second generation) to 2 × 105 (first generation) in the same 50 MRD-positive patients described above. Noteworthy, when only 2 × 105 cells (first generation) were analyzed up to 15 out of 50 MRD-positive cases (30%) were wrongly classified as being MRD negative because clonal PCs became undetectable or insufficient to define an MRD cluster (supplemental Figure 1, available on the Blood Web site). Furthermore, we showed that detecting persistent MRD with a sensitivity of 10−5 was clinically meaningful, since only MRD-negative cases (<10−5) had significantly longer survival, while patients with MRD levels between 10−4 and 10−5 had similar outcome to that of cases with MRD levels ≥10−4 (Figures 2D and 2E).
Improved specificity of MRD monitoring in MM of second- vs first-generation MFC. (A) PCA model for the phenotypic-based discrimination between normal (n = 17) BM PCs from healthy individuals and BM clonal PCs (n = 71) from MM patients. In the two-dimensional PCA plots, every healthy individual and patient is represented by a single dot and normal or MM reference PC groups by 1 (dashed lines) and 2 (solid lines) SD curves. Phenotypic makers are ordered according to their higher vs lower significance to discriminate between normal and clonal PCs. (B-C) Phenotypically selected clonal PCs from 50 MRD-positive MM patients (blue dots) were plotted against the PCA model based on all 8 phenotypic markers available with second-generation MFC (CD38, CD138, CD19, CD27, CD45, CD56, CD81, and CD117) vs the PCA model based on 4 phenotypic markers only, available with first-generation MFC (CD38, CD19 CD45 and CD56). (D-E) TTP (D) and OS (E) according to MRD status by second-generation MFC (n = 162). A total of 54 patients had undetectable MRD or MRD levels <0.001% (MRD−ve; <10−5), 20 cases had detectable MRD in between 0.001% and 0.02% (MRD+ve; ≥10−5 to <10−4), and the remaining 88 patients had detectable MRD at 0.01% or higher levels (MRD+ve; ≥10−4).
Improved specificity of MRD monitoring in MM of second- vs first-generation MFC. (A) PCA model for the phenotypic-based discrimination between normal (n = 17) BM PCs from healthy individuals and BM clonal PCs (n = 71) from MM patients. In the two-dimensional PCA plots, every healthy individual and patient is represented by a single dot and normal or MM reference PC groups by 1 (dashed lines) and 2 (solid lines) SD curves. Phenotypic makers are ordered according to their higher vs lower significance to discriminate between normal and clonal PCs. (B-C) Phenotypically selected clonal PCs from 50 MRD-positive MM patients (blue dots) were plotted against the PCA model based on all 8 phenotypic markers available with second-generation MFC (CD38, CD138, CD19, CD27, CD45, CD56, CD81, and CD117) vs the PCA model based on 4 phenotypic markers only, available with first-generation MFC (CD38, CD19 CD45 and CD56). (D-E) TTP (D) and OS (E) according to MRD status by second-generation MFC (n = 162). A total of 54 patients had undetectable MRD or MRD levels <0.001% (MRD−ve; <10−5), 20 cases had detectable MRD in between 0.001% and 0.02% (MRD+ve; ≥10−5 to <10−4), and the remaining 88 patients had detectable MRD at 0.01% or higher levels (MRD+ve; ≥10−4).
Clinical significance of MRD negativity in elderly MM patients
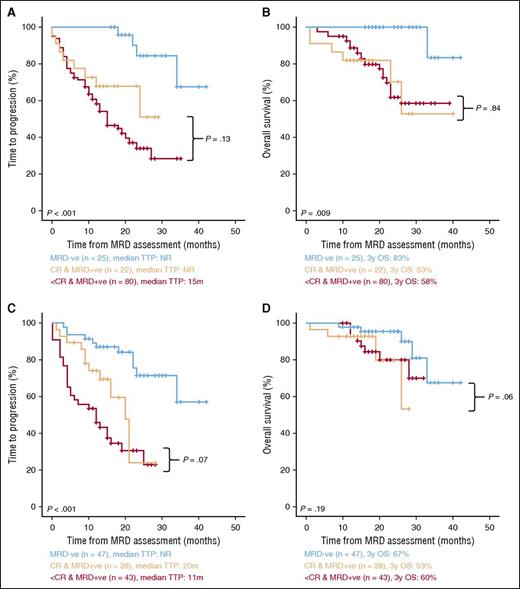
We first assessed the impact of the first 9 cycles of chemotherapy in the patients’ MRD status. Twenty-five of 127 cases (20%) monitored at cycle 9 were MRD negative, without significant differences between the sequential vs alternating regimens (20% vs 19%; P = .97). MRD-based stratification resulted in marked differences in outcome, with MRD-negative patients at cycle 9 showing significantly prolonged TTP and OS vs patients in complete remission (CR) but MRD positive and to those in less than CR (Figures 3A and 3B, respectively). In fact, no significant differences were observed between MRD-positive patients in CR and those in less than CR.
TTP and OS according to the depth of response of MM patients at cycles 9 and 18. (A-B) Cycle 9 (TTP and OS, respectively; n = 127) and (C-D) cycle 18 (TTP and OS, respectively; n = 118).
TTP and OS according to the depth of response of MM patients at cycles 9 and 18. (A-B) Cycle 9 (TTP and OS, respectively; n = 127) and (C-D) cycle 18 (TTP and OS, respectively; n = 118).
To understand the kinetics of MRD response with sequential vs alternating 18 cycles of treatment, we analyzed 83 patients with paired MRD assessments at cycles 9 and 18. Sixteen (19%) MRD-positive cases at cycle 9 became MRD negative at cycle 18, with no significant differences between rates of MRD negativity after sequential vs alternating regimens (23% vs 15%, respectively; P = .28). No MRD-negative cases at cycle 9 turned to MRD-positive cases at cycle 18. The overall MRD-negative rate at cycle 18 was slightly higher (but not significantly) in patients randomized to the sequential vs alternating schema (46% vs 33%; P = .16). Noteworthy, the design of the PETHEMA/GEM2010MAS65 trial allowed to investigate the immediate impact in patients’ outcome according to their MRD status without additional therapy. Thus, the median TTP from the moment of MRD assessment (cycle 18) was of only 12 months for patients in less than CR, 20 months for cases in CR but MRD positive, and not reached for the MRD-negative group (Figure 3C).
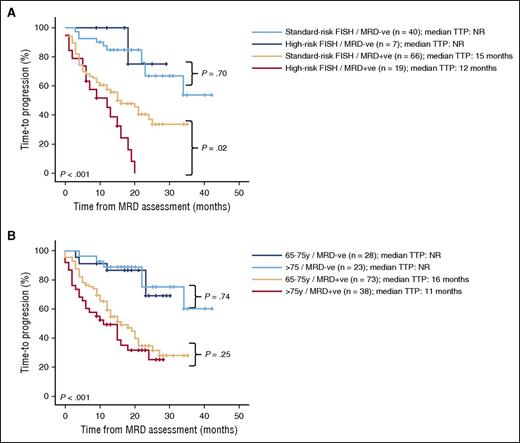
Afterward, we investigated the impact of MRD negativity among the cytogenetically defined standard- and high-risk subgroups. Noteworthy, high-risk patients attaining MRD negativity had significantly prolonged TTP vs MRD-positive patients and similar TTP to MRD-negative standard-risk cases; by contrast, MRD-positive patients with standard-risk cytogenetics had significantly inferior TTP, although their TTP was superior to that of high-risk MRD-positive cases (Figure 4A). We also investigated whether the impact of attaining MRD negativity was equally beneficial according to patients’ age. Interestingly, while median TTP from MRD assessment was not reached for patients aged 65-75 years and >75 years who reached MRD negativity, it became remarkably shorter for MRD-positive patients, irrespective of age (Figure 4B). These findings were similarly noted when patients’ MRD status was analyzed separately at cycles 9 and 18. Multivariate analysis including prognostic factors such as age, International Staging System stage, FISH cytogenetics, CR, and MRD response showed that only cytogenetics and MRD monitoring retained independent prognostic value for both TTP and OS (Table 1).
Impact of reaching MRD negativity on TTP according to patients' cytogenetic risk and age. (A) Cytogenetic risk (n = 132) and (B) age (n = 162).
Impact of reaching MRD negativity on TTP according to patients' cytogenetic risk and age. (A) Cytogenetic risk (n = 132) and (B) age (n = 162).
Multivariate analyses including baseline and posttreatment disease features with significant effect on TTP and/or OS in univariate analysis
| . | TTP . | OS . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| Age (<75 y vs ≥75 y) | — | — | 1.7 (0.8-3.7) | .16 |
| ISS (stage I vs II and III) | — | — | 2.0 (0.6-6.8) | .28 |
| Interphase FISH cytogenetics (standard vs high risk) | 2.0 (1.1-3.4) | .02 | 4.3 (2.0-9.2) | <.001 |
| Depth of response (CR vs <CR) | 1.7 (0.9-3.4) | .07 | 1.2 (0.5-2.8) | .63 |
| MRD (negative vs positive) | 2.7 (1.3-5.5) | .007 | 3.1 (1.1-8.8) | .04 |
| . | TTP . | OS . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P . | HR (95% CI) . | P . |
| Age (<75 y vs ≥75 y) | — | — | 1.7 (0.8-3.7) | .16 |
| ISS (stage I vs II and III) | — | — | 2.0 (0.6-6.8) | .28 |
| Interphase FISH cytogenetics (standard vs high risk) | 2.0 (1.1-3.4) | .02 | 4.3 (2.0-9.2) | <.001 |
| Depth of response (CR vs <CR) | 1.7 (0.9-3.4) | .07 | 1.2 (0.5-2.8) | .63 |
| MRD (negative vs positive) | 2.7 (1.3-5.5) | .007 | 3.1 (1.1-8.8) | .04 |
MRD status (negative vs positive) was determined at cycle 18 for the 118 out of the 162 patients with MRD assessment (Figure 1). Thus, the for the remaining 44 cases, the MRD status was determined at cycle 9, because no BM aspirates from these patients were centralized at cycle 18, typically because of disease progression (32%), toxicity (20%), withdrawal of informed consent (9%), or death (5%).
CI, confidence interval; ISS, International Staging System; high-risk FISH, t(4;14), t(14;16) and/or del(17p13).
Prognostic value of immune profiling during MRD monitoring
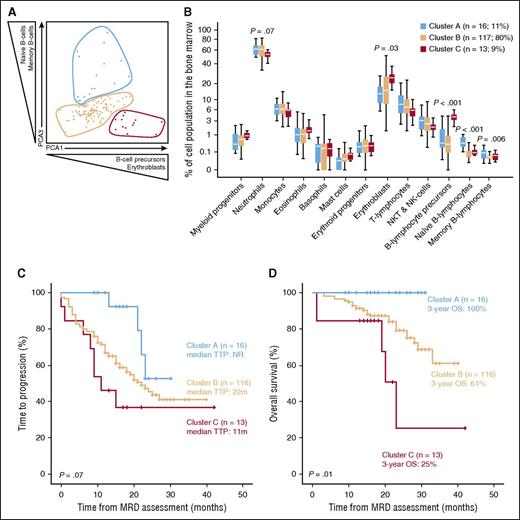
To evaluate whether the BM immune profile of individual patients at the time of MRD assessment could also be predictive of outcome, we developed individual immune signatures (n = 146) based on the unsupervised BM distribution of 13 immune cell populations identified with the second-generation MFC assay (n = 58 at cycle 9, n = 88 at cycle 18). This approach revealed the existence of 3 patient clusters (Figure 5A) (A, n = 16; B, n = 117; and C, n = 13) that were segregated by progressively increasing numbers of erythroblasts and B-cell precursors, together with progressively decreasing numbers of mature naïve and memory B cells (Figure 5B). There were no significant differences in cluster frequency according to treatment schema, baseline International Staging System stage, or FISH risk stratification. When compared with patients in clusters C and B, cases that clustered in group A had a trend toward a longer TTP (Figure 5C) and significantly superior OS (Figure 5D). Although the numbers preclude a definitive conclusion, a similar trend in patients’ outcome according to their immune profile was observed when separately analyzed at cycles 9 and 18. Noteworthy, there were no significant differences according to patients’ MRD status across the 3 clusters; thus, even among MRD-positive patients, immune profiling continued to show an impact on patient survival, with 3-year OS rates of 100%, 65%, and 0% for clusters A, B, and C, respectively (P = .003).
Prognostic value of immune profiling using second-generation MFC. (A) Two-dimensional PCA plot of the patients’ immune profiles defined by the distribution of 13 immune cell populations (excluding normal and clonal PCs) in the BM at the time of MRD assessment (n = 146). (B-C) The distribution of the 13 immune cell populations in clusters A (n = 16), B (n = 116), and C (n = 13) is shown in panel B, whereas in panel C shows TTP and OS according to the patients’ immune-profile clusters. NK, natural killer; NKT, natural killer T cells.
Prognostic value of immune profiling using second-generation MFC. (A) Two-dimensional PCA plot of the patients’ immune profiles defined by the distribution of 13 immune cell populations (excluding normal and clonal PCs) in the BM at the time of MRD assessment (n = 146). (B-C) The distribution of the 13 immune cell populations in clusters A (n = 16), B (n = 116), and C (n = 13) is shown in panel B, whereas in panel C shows TTP and OS according to the patients’ immune-profile clusters. NK, natural killer; NKT, natural killer T cells.
Discussion
Over the last decade, different groups have shown the added value of MRD assessment over conventional response criteria in transplant-eligible MM patients.16,17,19,26-28 MRD clearance is also achievable in elderly MM patients in the era of novel and more effective treatment strategies,15-17,19-21 but because its prognostic value has only been sporadically investigated in well-selected transplant-ineligible patients,15,19 its potential role as a biomarker to predict survival remains less clear in elderly MM patients. Herein, we show that on intention to treat, up to 22% (n = 54/241) of transplant-ineligible patients enrolled in the PETHEMA/GEM2012MAS65 study reached MRD negativity, which resulted in a significant prolongation in TTP and OS. Similarly to what has been previously postulated for transplant candidates,11,28 MRD response emerged here as one of the most relevant prognostic factors in elderly MM patients.
Most of the available data on the prognostic value of flow-based MRD assessment were obtained using conventional, “first generation” MFC based in 4- or 6-color combinations, with a limit of detection of 10−4.29 More sophisticated, “second generation” MFC has been progressively introduced21,26 and is expected to improve the sensitivity and specificity of MRD monitoring, but the extent of such improvement has never been investigated. Here, we used the cytometric software developed by the EuroFlow Consortium24,30 to show that the transition from a first-generation 4-color to a second-generation 8-color MFC assay that measured 10 times more cells resulted in a significantly increased specificity and sensitivity. Noteworthy, we showed that by applying the limit of detection reached with first-generation MFC (ie, 10−4), up to 30% of patients with persistent MRD detectable by second-generation MFC would had been wrongly classified as MRD negative. We also showed that the ability to monitor MRD up to the 10−5 sensitivity level is clinically relevant, because this level identifies a subset of patients (those between 10−4 and 10−5) with inferior survival than MRD-negative (<10−5) cases and similar to that of MRD-positive patients at the ≥10−4 level. Our results extent on recent data reported by Korde et al,21 in which the prognostic value of MRD monitoring using novel 8-color MFC compares well to that of next-generation sequencing (NGS) (1 relapse among MRD-negative cases by MFC vs 0 relapses among MRD-negative cases by NGS) and shows superior intention-to-treat applicability (98% vs 80% for MFC vs NGS, respectively).21 That notwithstanding, the advent of even more sensitive “next-generation” MFC will likely outperform the method used in the present study31 and, therefore, the ability of MFC to monitor MRD and predict survival will continue to increase in MM. The same applies for the advent of more sensitive and applicable NGS as compared with former molecular methods.17,21 Accordingly, the recent development and availability of 2 highly-sensitive and potentially standardized next-generation methods envisions that MRD monitoring and patient prognostication will be even more powerful in the future.
In the present study, we have shown that sensitive MRD assessment after the first 9 cycles of chemotherapy allowed us to discriminate among patients with remarkably different outcomes; thus, only 16% of MRD-negative patients at cycle 9 have progressed so far, whereas more than half (54%) of MRD-positive cases have relapsed despite receiving further chemotherapy. Noteworthy, no significant differences were observed between MRD-positive patients in CR vs less than CR, suggesting that current response categories fail to identify patients with different outcomes if MRD persists. Even among patients in CR plus a normal serum free light chain ratio, the persistence of MRD predicted significantly inferior TTP (data not shown). Furthermore, because MRD-positive cases at cycle 9 had identically dismal outcomes despite receiving 9 additional cycles of Rd or VMP/Rd (sequential or alternating scheme, respectively; data not shown), they might be considered as candidates for novel agents with alternative mechanisms of action (eg, monoclonal antibodies).1,2 It should be noted that in contrast to previous studies in which MRD assessment was performed at intermediate stages of patients’ treatment (eg, before maintenance),15 the design of the PETHEMA/GEM2010MAS65 trial allowed to investigate the immediate impact on patients’ outcome according to their MRD status without additional therapy. Thus, we report here new data showing that MRD-positive patients at cycle 18 (ie, without further therapy) had a TTP after MRD assessment of ∼1.5 years without statistically significant differences according to conventional response criteria (ie, CR vs less than CR). The clinical significance of our results is twofold: (1) MRD-positive patients should be considered as candidates for further (alternative) therapies in order to control chemoresistant PCs, and (2) the definition of CR would also benefit elderly patients by incorporating MRD assessment into the response criteria.11 In this regard, sequential MRD monitoring would be particularly attractive to identify patients with sustained MRD negativity; accordingly, herein, the best outcome was noted among the 18 MRD-negative cases at both cycles 9 and 18, 15 patients (ie, 83%) remain progression-free and 17 (ie, 94%) are alive, despite no additional therapy.
Due to their poor prognosis and the unmet need for novel agents, patients with high-risk cytogenetics are ideal candidates to investigate the role of MRD monitoring both as a clinical end point for novel treatment modalities and a surrogate biomarker for survival. Here, we show that patients with high-risk FISH abnormalities reaching MRD negativity may experience a TTP similar to that of MRD-negative cases and standard-risk cytogenetics; by contrast, TTP of standard-risk MRD-positive patients was slightly but significantly (P = .02) superior to that of high-risk MRD-positive cases, highlighting the independent and complementary role of cytogenetic and MRD risk stratification in elderly MM patients. Another interesting finding reported here is the fact that reaching MRD negativity equally benefited elderly patients older than 75 years. These observations suggest that eradication of MRD might be considered as a clinical end point for all elderly patients, providing the tolerability of the proposed treatment strategy.
Recently, Barlogie et al have shown that the vast majority of CR patients achieving long-term survival (10-years relapse-free) were also MRD negative.7 However, attaining deep-remission is not a prerequisite to achieving long-term disease control,7,32 at least in specific cases, and more accurate identification of such patients should also become a research priority. Here, we show for the first time that immune profiling in MM after therapy, in parallel to MRD monitoring, might be prognostically relevant by allowing the identification of patients with either poor survival or sustained disease control. Accordingly, flow-based MRD monitoring offers complementary information to the quantification of MRD levels, and may contribute to identify a subset of patients that albeit being MRD-positive can still experience prolonged survival due to a unique immune signature specifically characterized by a more prominent regeneration of mature B lymphocytes. In fact, a similar immune signature was previously found in both MRD-negative and positive MM patients reaching long-term disease control.33
In summary, here we show that second-generation MFC supersedes previous flow-based MRD monitoring by identifying patients with lower MRD levels (<10−4) and poor outcome, as well as MRD-positive cases with prolonged survival associated with a unique immune profiling at the time of response assessment. We also revealed that similarly to transplant candidates, MRD monitoring is one of the most relevant prognostic factors in elderly MM patients, complimentary to the cytogenetic risk and superior to conventional response criteria; thus, patients with standard-risk MM and those in CR but remaining MRD positive experience poor outcomes and warrant potential treatment individualization to improve their survival. The availability of highly effective therapies for elderly MM patients urges the need to address if response-driven (ie, MRD-based) treatment decisions can reduce the difference in survival between transplant-eligible and elderly patients (or even standard- vs high-risk MM); this requires a cooperative effort toward novel clinical trial designs in which patients are accurately stratified according to sensitive MRD monitoring prior to alternative treatment strategies, or even randomized into different therapeutic approaches according to MRD status. Such clinical trials are needed to establish the exact role of MRD testing in elderly MM patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all members of the Spanish Myeloma Group.
This study was supported by Cooperative Research Thematic Network grants RD12/0036/0058, RD12/0036/0048, RD12/0036/0046, and RD12/0036/0061 of the Red de Cancer (Cancer Network of Excellence); Instituto de Salud Carlos III, Spain, Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria (FIS: PI060339; 06/1354; 02/0905; 01/0089/01-02; PS09/01897/01370; PI13/01469, PI14/01867, G03/136; Sara Borrell: CD13/00340 and CD12/00540); and Asociación Española Contra el Cáncer (GCB120981SAN). The study was also supported internationally by the Black Swan Research Initiative of the International Myeloma Foundation and a European Research Council 2015 starting grant.
Authorship
Contribution: B.P., M.-V.M., J.J.L., and J.F.S.M. were responsible for study conception and design; B.P., M.-T.C., N.P., P.A., M.-B.V., L.C., J.F.-M., N.C.G., M.-L.M.-R., J.J.M.V.D., and A. Orfao developed study methodology; B.P., M.-T.C., N.P., P.A., M.-B.V., L.C., J.F.-M., N.C.G., M.-L.M.-R., J.M.-L., E.M.O., M.T.H., A.-I.T., L.R., M.-A.E., R.M., M.G., A. Oriol, C.C., J.M., J. Bargay, C.E., Y.G., J. Bladé, M.-V.M., J.J.L., and J.F.S.M. acquired data; B.P., P.A., M.-V.M., J.J.L., and J.F.S.M. analyzed and interpreted data; B.P. and J.F.S.M. wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: B.P. has received consulting fees from Celgene and Janssen and research support from Celgene. E.M.O. discloses research funding, honoraria, and consultancy from Celgene and honoraria and consultancy from Janssen. L.R. received grant support from Janssen. A. Oriol has served as member of advisory boards for Janssen and Celgene. J.B. received grant support from Janssen and Celgene. M.-V.M. has served on the speaker’s bureau and as member of advisory boards for Celgene and Janssen. J.F.S.M. has received consulting fees from Celgene and Janssen.
A complete list of the members of the Grupo Español de Mieloma/Programa para el Estudio de la Terapéutica en Hemopatías Malignas (GEM/PETHEMA) Cooperative Study Groups appears in “Appendix.”
Correspondence: Jesus F. San Miguel, Clinica Universidad de Navarra, Centro de Investigacion Médica Aplicada (CIMA), Av Pio XII 36, 31008 Pamplona, Spain; e-mail: sanmiguel@unav.es.
Appendix: study group members
The members of the Grupo Español de Mieloma/Programa para el Estudio de la Terapéutica en Hemopatías Malignas (GEM/PETHEMA) Cooperative Study Groups are: Ernesto Pérez Persona, Antonia Sampol Mayol, Joan Bargay Lleonart, Eugenia Abella Monreal, Joan Bladé Creixentí, Miguel Granell Gorrochategui, Albert Oriol Rocafiguera, Albert Altes Hernández, Elena Rámila Herrero, Mercedes Gironella Mesa, Anna Sureda Balari, Carmen Cabrera Silva, Francisco Javier Capote Huelva, José Luis Guzmán Zamudio, María Mas Esteve, Carmen Calle Primo, José Luis Bello López, Carlos Cerveró Santiago, Yolanda González Montes, Dunia de Miguel Llorente, María Asunción Echeveste Gutiérrez, Fernando Escalante Barrigón, Raquel de Paz Arias, María Jesús Blanchard Rodríguez, Juan José Lahuerta Palacios, Isabel Krsnik Castello, Rafael Martínez Martínez, Adrián Alegre Amor, Cristina Encinas Rodríguez, Juan José Gil Fernández, Carolina Bombín Canal, Pilar Bravo Barahona, Francisco Javier Peñalver Párraga, Rebeca Iglesias del Barrio, Jaime Pérez de Oteyza, Eugenio Giménez Mesa, José Ángel Hernández Rivas, Ana Paz Lafuente Guijosa, María Casanova Espinosa, José María Moraleda Jiménez, Felipe de Arriba de la Fuente, María Ángeles Goñi Herranz, Felipe Prósper Cardoso, Jesús F. San Miguel Izquierdo, Alexia Suárez Cabrera, Marivi Mateos Mateos, Miguel Teodoro Hernández García, Eulogio Conde García, José Mariano Hernández Martín, Eduardo Ríos Herranz, Jesús Martín Sánchez, José Luis Bueno Cabrera, Felipe Casado Montero, Javier de la Rubia Comos, Paz Ribas García, Aurelio López Martínez, Ana Isabel Teruel Casasus, María Ángeles Ruíz Guinaldo, Elena Amutio Díez, Monserrat Pérez Sánchez, Luis Palomera Bernal, and Pilar Delgado Beltrán.