Abstract
Mycosis fungoides (MF) is the most common primary cutaneous T-cell lymphoma variant and is closely related to a rare leukemic variant, Sézary syndrome (SS). MF patients at risk of disease progression can now be identified and an international consortium has been established to address the prognostic relevance of specific biologic factors and define a prognostic index. There are a lack of randomized clinical trial data in MF/SS and evidence is based on a traditional “stage-based” approach; treatment of early-stage disease (IA-IIA) involves skin directed therapies which include topical corticosteroids, phototherapy (psoralen with UVA or UVB), topical chemotherapy, topical bexarotene, and radiotherapy including total skin electron beam therapy. Systemic approaches are used for refractory early-stage and advanced-stage disease (IIB-IV) and include bexarotene, interferon α, extracorporeal photopheresis, histone deacetylase inhibitors, and antibody therapies such as alemtuzumab, systemic chemotherapy, and allogeneic transplantation. However, despite the number of biologic agents available, the treatment of advanced-stage disease still represents an unmet medical need with short duration of responses. Encouragingly, randomized phase 3 trials are assessing novel agents, including brentuximab vedotin and the anti-CCR4 antibody, mogamulizumab. A broader understanding of the biology of MF/SS will hopefully identify more effective targeted therapies.
Introduction
Primary cutaneous lymphomas represent rare extranodal lymphomas defined by specific clinicopathologic features.1-3 This review focuses on mycosis fungoides (MF) and its leukemic variant, Sézary syndrome (SS). Published treatment guidelines include the National Comprehensive Cancer Network (NCCN) guidelines (www.nccn.org),4 the European Society of Medical Oncology (ESMO),5 and the European Organization of Research and Treatment of Cancer (EORTC),6 but there is a restricted evidence base as there are few randomized trials.7 The management approach is truly multidisciplinary. Treatment options are outlined in Tables 1 and 2.
Summary of treatment options for MF/SS
| Therapy . | MF . | SS/E-MF . | Comment including potential toxicities . | |
|---|---|---|---|---|
| Early-stage disease . | Advanced-stage disease . | |||
| “Expectant policy” | ++ | Usually suitable for those with stage IA disease in conjunction with symptomatic treatment if required. Patients with single lesion may be considered for “curative therapy” with RT. | ||
| Topical corticosteroids | ++++ | ++ | +++ | Simple therapy. Toxicities if extensive skin application for long periods. |
| PUVA | +++ | + | +++ | For patch/plaque disease. Requires regular 2-3×/wk treatment. There may be limited availability of PUVA in nonmetropolitan areas. Can be combined with retinoids/rexinoids. Risk of skin cancers with cumulative dosing. |
| UVB (TLO1) | ++++ | + | ++ | For patch stage disease as skin penetration not as deep as PUVA. Requires regular 2-3×/wk treatment and generally more readily available than PUVA. Risk of skin cancers with cumulative dosing. |
| Topical chemotherapy | ++ | If limited number of lesions. For limited sites of disease. Local reactions occasionally problematic. | ||
| Imiquimod | + | If small lesions and limited number of lesions. Can cause inflammatory reactions. | ||
| Photodynamic therapy | + | If limited number of lesions. Limited availability. | ||
| Retinoids | + | + | + | Usually second line. Less used since bexarotene became available. |
| Bexarotene | ++ | +++ | ++++ | Usually second line. Generally well tolerated and convenient (oral capsule). Some responses can be very durable. Most common side effects are hypertriglyceridemia and hypothyroidism that usually require treatment. Other relatively common side effects are rash and headache. Can be used in conjunction with other therapies such as PUVA or IFN-α and ECP for E-MF/SS. Topical form for limited sites of disease but local reactions may occur. |
| Interferon α | ++ | +++ | ++++ | Second line; major difficulty is tolerance and compliance. Some responses can be very durable. Somewhat inconvenient (daily sc injection). Most common side effect is fatigue, anorexia, and mood changes particularly in older patients. Monitoring for cytopenias and thyroid disturbance is recommended. Requires moderately high doses aiming for 3-5+ MU/d or 3×/wk. Monitor FBC and thyroid function. IFN-α can also be combined with PUVA, retinoids, bexarotene. |
| HDACi: vorinostat, romidepsin | ++ | +++ | ++++ | Beyond second line. Most common SEs are fatigue, lethargy, mild/moderate thrombocytopenia, and elevated creatinine and taste changes. Can improve itch even when skin lesions remain. Some responses can be very durable. Limited data on use in combination with other therapies. |
| Oral methotrexate | + | +++ | +++ | Low dose weekly. Generally well tolerated and convenient (oral weekly). Dose-response effect is common and usually start at 20-30 mg/wk (up to 60-70 mg/wk). Some responses can be very durable. Most common side effects are cytopenias and long-term risk of liver disease. Very effective in patients with coexistent lymphomatoid papulosis. Can be used in conjunction with other therapies such as steroids, ECP, PUVA, IFN-α. |
| Localized radiotherapy | +++ | +++ | If localized or large/plaques and tumor nodules. | |
| TSEB | + | ++ | + | For widespread disease. Higher doses associated with acute skin toxicities. Can be repeated but high cumulative doses can result in skin toxicity. |
| Systemic chemotherapy | ++ | ++ | Beyond second line. | |
| ECP | ++++ | Variable availability. Venous access can be problematic. | ||
| Pralatrexate | +++ | + | Beyond second line. Generally well tolerated. Mucositis is most common toxicity. Supportive measures with B12 supplementation required. Dose modification generally recommended for MF (lower dose than PTCL). | |
| Allogeneic transplantation | + | ++ | Very selected cases. | |
| Denileukin diftitox | ++ | ++ | Beyond second line (availability limited currently). Major toxicity is capillary leak syndrome which can be severe. | |
| Alemtuzumab | + | ++ | Second line or beyond. More effective in SS/E-MF. Higher doses are associated with CMV reactivation. | |
| Immunomodulatory agents (lenalidomide) | + | Beyond second line. Has not achieved regulatory approval. Fatigue, venous thromboembolism, and cytopenias are the most common toxicities. | ||
| Proteasome inhibitors | + | Under investigation. Has not achieved regulatory approval. Cytopenias and neuropathy (bortezomib) can occur. | ||
| Novel agents and clinical trials | Chemotherapy generally only achieves short remissions and clinical trials/novel agents can be recommended before chemotherapy is considered. | |||
| Therapy . | MF . | SS/E-MF . | Comment including potential toxicities . | |
|---|---|---|---|---|
| Early-stage disease . | Advanced-stage disease . | |||
| “Expectant policy” | ++ | Usually suitable for those with stage IA disease in conjunction with symptomatic treatment if required. Patients with single lesion may be considered for “curative therapy” with RT. | ||
| Topical corticosteroids | ++++ | ++ | +++ | Simple therapy. Toxicities if extensive skin application for long periods. |
| PUVA | +++ | + | +++ | For patch/plaque disease. Requires regular 2-3×/wk treatment. There may be limited availability of PUVA in nonmetropolitan areas. Can be combined with retinoids/rexinoids. Risk of skin cancers with cumulative dosing. |
| UVB (TLO1) | ++++ | + | ++ | For patch stage disease as skin penetration not as deep as PUVA. Requires regular 2-3×/wk treatment and generally more readily available than PUVA. Risk of skin cancers with cumulative dosing. |
| Topical chemotherapy | ++ | If limited number of lesions. For limited sites of disease. Local reactions occasionally problematic. | ||
| Imiquimod | + | If small lesions and limited number of lesions. Can cause inflammatory reactions. | ||
| Photodynamic therapy | + | If limited number of lesions. Limited availability. | ||
| Retinoids | + | + | + | Usually second line. Less used since bexarotene became available. |
| Bexarotene | ++ | +++ | ++++ | Usually second line. Generally well tolerated and convenient (oral capsule). Some responses can be very durable. Most common side effects are hypertriglyceridemia and hypothyroidism that usually require treatment. Other relatively common side effects are rash and headache. Can be used in conjunction with other therapies such as PUVA or IFN-α and ECP for E-MF/SS. Topical form for limited sites of disease but local reactions may occur. |
| Interferon α | ++ | +++ | ++++ | Second line; major difficulty is tolerance and compliance. Some responses can be very durable. Somewhat inconvenient (daily sc injection). Most common side effect is fatigue, anorexia, and mood changes particularly in older patients. Monitoring for cytopenias and thyroid disturbance is recommended. Requires moderately high doses aiming for 3-5+ MU/d or 3×/wk. Monitor FBC and thyroid function. IFN-α can also be combined with PUVA, retinoids, bexarotene. |
| HDACi: vorinostat, romidepsin | ++ | +++ | ++++ | Beyond second line. Most common SEs are fatigue, lethargy, mild/moderate thrombocytopenia, and elevated creatinine and taste changes. Can improve itch even when skin lesions remain. Some responses can be very durable. Limited data on use in combination with other therapies. |
| Oral methotrexate | + | +++ | +++ | Low dose weekly. Generally well tolerated and convenient (oral weekly). Dose-response effect is common and usually start at 20-30 mg/wk (up to 60-70 mg/wk). Some responses can be very durable. Most common side effects are cytopenias and long-term risk of liver disease. Very effective in patients with coexistent lymphomatoid papulosis. Can be used in conjunction with other therapies such as steroids, ECP, PUVA, IFN-α. |
| Localized radiotherapy | +++ | +++ | If localized or large/plaques and tumor nodules. | |
| TSEB | + | ++ | + | For widespread disease. Higher doses associated with acute skin toxicities. Can be repeated but high cumulative doses can result in skin toxicity. |
| Systemic chemotherapy | ++ | ++ | Beyond second line. | |
| ECP | ++++ | Variable availability. Venous access can be problematic. | ||
| Pralatrexate | +++ | + | Beyond second line. Generally well tolerated. Mucositis is most common toxicity. Supportive measures with B12 supplementation required. Dose modification generally recommended for MF (lower dose than PTCL). | |
| Allogeneic transplantation | + | ++ | Very selected cases. | |
| Denileukin diftitox | ++ | ++ | Beyond second line (availability limited currently). Major toxicity is capillary leak syndrome which can be severe. | |
| Alemtuzumab | + | ++ | Second line or beyond. More effective in SS/E-MF. Higher doses are associated with CMV reactivation. | |
| Immunomodulatory agents (lenalidomide) | + | Beyond second line. Has not achieved regulatory approval. Fatigue, venous thromboembolism, and cytopenias are the most common toxicities. | ||
| Proteasome inhibitors | + | Under investigation. Has not achieved regulatory approval. Cytopenias and neuropathy (bortezomib) can occur. | ||
| Novel agents and clinical trials | Chemotherapy generally only achieves short remissions and clinical trials/novel agents can be recommended before chemotherapy is considered. | |||
Crosses indicate approximate frequency of use.
CMV, cytomegalovirus; FBC, full blood count; PTCL, peripheral T-cell lymphoma; RT, radiotherapy; sc, subcutaneous; SE, side effects.
Recommendations for first-line treatment of stage III or SS (stages III or IVA)
| Treatment . | Comments . |
|---|---|
| First-line | |
| ECP | Well tolerated with limited toxicities. Circulating T-cell clone should be detectable in blood by either morphology, flow cytometry, or molecular studies. Should not be considered in patients with SS who have extensive nodal (IVa) or visceral (IVb) disease. Side effects to methoxsalen is rare. Requires good venous access with the associated risk of infection. Often combined with oral steroids (short-term), IFN-α, bexarotene, or low-dose MTX. Improvement with ECP alone can take some weeks and maximum improvement may not be seen for many months. Durable responses are not uncommon. |
| IFN-α | Major difficulty is tolerance and compliance. Some responses can be very durable. Somewhat inconvenient (daily sc injection). Most common side effect is fatigue particularly in older patients. Requires moderately high doses aiming for 3-5+ MU/d. Monitor FBC and thyroid function. IFN-α can also be combined with PUVA, retinoids, bexarotene, and ECP. |
| PUVA + IFN-α | For stage III disease. Would not generally recommend PUVA alone. Requires regular 2-3×/wk treatment and limited number of sites in nonmetropolitan areas. |
| Bexarotene | See Table 1 for comments. Can consider adding to ECP or IFN-α. |
| MTX | See Table 1 for comments. |
| Second-line | |
| Alemtuzumab | See Table 1 for comments. Can be considered first line in suitable patients. |
| Vorinostat/romidepsin | See Table 1 for comments. No data available of adding to ECP or IFN-α. |
| Pralatrexate | See Table 1 for comments. |
| Allogeneic transplant | For suitable patients. |
| Novel agents and clinical trials | In patients with SS, chemotherapy is recommended after bexarotene and/or and HDACi and/or DD. It is very acceptable to consider a clinical trials/novel agents before chemotherapy is considered. |
| Chemotherapy | Choice of chemotherapy regimens is extensive and choice depends on patient tolerance, risk of infection vs the relatively short duration of remission observed with most chemotherapy regimens. Transplantation may be considered in highly selected individuals. |
| Treatment . | Comments . |
|---|---|
| First-line | |
| ECP | Well tolerated with limited toxicities. Circulating T-cell clone should be detectable in blood by either morphology, flow cytometry, or molecular studies. Should not be considered in patients with SS who have extensive nodal (IVa) or visceral (IVb) disease. Side effects to methoxsalen is rare. Requires good venous access with the associated risk of infection. Often combined with oral steroids (short-term), IFN-α, bexarotene, or low-dose MTX. Improvement with ECP alone can take some weeks and maximum improvement may not be seen for many months. Durable responses are not uncommon. |
| IFN-α | Major difficulty is tolerance and compliance. Some responses can be very durable. Somewhat inconvenient (daily sc injection). Most common side effect is fatigue particularly in older patients. Requires moderately high doses aiming for 3-5+ MU/d. Monitor FBC and thyroid function. IFN-α can also be combined with PUVA, retinoids, bexarotene, and ECP. |
| PUVA + IFN-α | For stage III disease. Would not generally recommend PUVA alone. Requires regular 2-3×/wk treatment and limited number of sites in nonmetropolitan areas. |
| Bexarotene | See Table 1 for comments. Can consider adding to ECP or IFN-α. |
| MTX | See Table 1 for comments. |
| Second-line | |
| Alemtuzumab | See Table 1 for comments. Can be considered first line in suitable patients. |
| Vorinostat/romidepsin | See Table 1 for comments. No data available of adding to ECP or IFN-α. |
| Pralatrexate | See Table 1 for comments. |
| Allogeneic transplant | For suitable patients. |
| Novel agents and clinical trials | In patients with SS, chemotherapy is recommended after bexarotene and/or and HDACi and/or DD. It is very acceptable to consider a clinical trials/novel agents before chemotherapy is considered. |
| Chemotherapy | Choice of chemotherapy regimens is extensive and choice depends on patient tolerance, risk of infection vs the relatively short duration of remission observed with most chemotherapy regimens. Transplantation may be considered in highly selected individuals. |
The diagnosis of MF/SS is based on an assessment of clinicopathologic criteria which distinguish MF/SS from rarer subtypes. A consensus approach to the pathological diagnosis of early-stage MF has been proposed by the International Society of Cutaneous Lymphoma (ISCL).8 Repeated skin biopsies are often required but the diagnosis of erythrodermic MF (E-MF) is one of the most challenging in dermatologic practice. An updated staging system proposed by the ISCL/EORTC has been adopted by the American Joint Committee on Cancer (Tables 3 and 4).9,10 For patients with clinical stage IA-IB and no palpable lymphadenopathy, extensive staging investigations are not required. Appropriate investigations are listed in Table 5. Core or excisional biopsies of suspicious lymph nodes are required to provide an accurate pathologic assessment; fine needle aspiration assessment is generally inadequate. Bone marrow trephine biopsies are usually not required unless there are unexplained hematologic abnormalities. Almost two-thirds of patients present with stage IA-IIA and, although patients with early stages of MF have an excellent prognosis, ∼25% develop progressive disease.11-13
ISCL/EORTC revision to the classification of MF and SS
| TNMB classification . | . |
|---|---|
| Skin | |
| T1 | Limited patches,* papules, and/or plaques† covering <10% of the skin surface. May further stratify into T1a (patch only) vs T1b (plaque ± patch). |
| T2 | Patches, papules, or plaques covering ≥10% of the skin surface. May further stratify into T2a (patch only) vs T2b (plaque ± patch). |
| T3 | One or more tumors‡ (≥1-cm diameter). |
| T4 | Confluence of erythema covering ≥80% BSA. |
| Node | |
| N0 | No clinically abnormal peripheral lymph nodes§; biopsy not required. |
| N1 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 1 or NCI LN0-2. |
| N1a | Clone negative.|| |
| N1b | Clone positive.|| |
| N2 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 2 or NCI LN3. |
| N2a | Clone negative.|| |
| N2b | Clone positive.|| |
| N3 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grades 3-4 or NCI LN4; clone positive or negative. |
| Nx | Clinically abnormal peripheral lymph nodes; no histologic confirmation. |
| Visceral | |
| M0 | No visceral organ involvement. |
| M1 | Visceral involvement (must have pathology confirmation¶ and organ involved should be specified). |
| Blood | |
| B0 | Absence of significant blood involvement: ≤5% of peripheral blood lymphocytes are atypical (Sézary) cells.# |
| B0a | Clone negative.|| |
| B0b | Clone positive.|| |
| B1 | Low blood tumor burden: >5% of peripheral blood lymphocytes are atypical (Sézary) cells but does not meet the criteria of B2. |
| B1a | Clone negative.|| |
| B1b | Clone positive.|| |
| B2 | High blood tumor burden: ≥1000/µL Sézary cells# with positive clone.|| |
| TNMB classification . | . |
|---|---|
| Skin | |
| T1 | Limited patches,* papules, and/or plaques† covering <10% of the skin surface. May further stratify into T1a (patch only) vs T1b (plaque ± patch). |
| T2 | Patches, papules, or plaques covering ≥10% of the skin surface. May further stratify into T2a (patch only) vs T2b (plaque ± patch). |
| T3 | One or more tumors‡ (≥1-cm diameter). |
| T4 | Confluence of erythema covering ≥80% BSA. |
| Node | |
| N0 | No clinically abnormal peripheral lymph nodes§; biopsy not required. |
| N1 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 1 or NCI LN0-2. |
| N1a | Clone negative.|| |
| N1b | Clone positive.|| |
| N2 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grade 2 or NCI LN3. |
| N2a | Clone negative.|| |
| N2b | Clone positive.|| |
| N3 | Clinically abnormal peripheral lymph nodes; histopathology Dutch grades 3-4 or NCI LN4; clone positive or negative. |
| Nx | Clinically abnormal peripheral lymph nodes; no histologic confirmation. |
| Visceral | |
| M0 | No visceral organ involvement. |
| M1 | Visceral involvement (must have pathology confirmation¶ and organ involved should be specified). |
| Blood | |
| B0 | Absence of significant blood involvement: ≤5% of peripheral blood lymphocytes are atypical (Sézary) cells.# |
| B0a | Clone negative.|| |
| B0b | Clone positive.|| |
| B1 | Low blood tumor burden: >5% of peripheral blood lymphocytes are atypical (Sézary) cells but does not meet the criteria of B2. |
| B1a | Clone negative.|| |
| B1b | Clone positive.|| |
| B2 | High blood tumor burden: ≥1000/µL Sézary cells# with positive clone.|| |
NCI LN, National Cancer Institute Lymph Node; PCR, polymerase chain reaction; TCR, T-cell receptor; TNMB, tumor-node-metastasis-blood.
For skin, patch indicates any size skin lesion without significant elevation or induration. Presence/absence of hypo- or hyperpigmentation, scale, crusting, and/or poikiloderma should be noted.
For skin, plaque indicates any size skin lesion that is elevated or indurated. Presence or absence of scale, crusting, and/or poikiloderma should be noted. Histologic features such as folliculotropism or large-cell transformation (>25% large cells), CD30+ or CD30−, and clinical features such as ulceration are important to document.
For skin, tumor indicates at least one 1-cm diameter solid or nodular lesion with evidence of depth and/or vertical growth. Note total number of lesions, total volume of lesions, largest size lesion, and region of body involved. Also note if histologic evidence of large-cell transformation has occurred. Phenotyping for CD30 is encouraged.
For node, abnormal peripheral lymph node(s) indicates any palpable peripheral node that on physical examination is firm, irregular, clustered, fixed, or 1.5 cm or larger in diameter. Node groups examined on physical examination include cervical, supraclavicular, epitrochlear, axillary, and inguinal. Central nodes, which are not generally amenable to pathologic assessment, are not currently considered in the nodal classification unless used to establish N3 histopathologically.
A T-cell clone is defined by PCR or Southern blot analysis of the T-cell receptor gene.
For viscera, spleen and liver may be diagnosed by imaging criteria.
For blood, Sézary cells are defined as lymphocytes with hyperconvoluted cerebriform nuclei. If Sézary cells are not able to be used to determine tumor burden for B2, then 1 of the following modified ISCL criteria along with a positive clonal rearrangement of the TCR may be used instead: (1) expanded CD4+ or CD3+ cells with CD4/CD8 ratio of 10 or more, (2) expanded CD4+ cells with abnormal immunophenotype including loss of CD7 or CD26.
ISCL/EORTC revision to the staging of MF and SS
| . | T . | N . | M . | B . |
|---|---|---|---|---|
| IA | 1 | 0 | 0 | 0,1 |
| IB | 2 | 0 | 0 | 0,1 |
| IIA | 1,2 | 1,2 | 0 | 0,1 |
| IIB* | 3 | 0-2 | 0 | 0,1 |
| III* | 4 | 0-2 | 0 | 0,1 |
| IIIA* | 4 | 0-2 | 0 | 0 |
| IIIB* | 4 | 0-2 | 0 | 1 |
| IVA1* | 1-4 | 0-2 | 0 | 2 |
| IVA2* | 1-4 | 3 | 0 | 0-2 |
| IVB* | 1-4 | 0-3 | 1 | 0-2 |
| . | T . | N . | M . | B . |
|---|---|---|---|---|
| IA | 1 | 0 | 0 | 0,1 |
| IB | 2 | 0 | 0 | 0,1 |
| IIA | 1,2 | 1,2 | 0 | 0,1 |
| IIB* | 3 | 0-2 | 0 | 0,1 |
| III* | 4 | 0-2 | 0 | 0,1 |
| IIIA* | 4 | 0-2 | 0 | 0 |
| IIIB* | 4 | 0-2 | 0 | 1 |
| IVA1* | 1-4 | 0-2 | 0 | 2 |
| IVA2* | 1-4 | 3 | 0 | 0-2 |
| IVB* | 1-4 | 0-3 | 1 | 0-2 |
B, blood; M, metastasis; N, node; T, tumor.
Considered “advanced-stage” disease.
Recommended evaluation/initial staging of the patient with MF/SS
| Recommended evaluation/initial staging . |
|---|
| Complete physical examination including |
| Determination of type(s) of skin lesions. |
| If only patch/plaque disease or erythroderma, then estimate percentage of BSA involved and note any ulceration of lesions. |
| If tumors are present, determine total number of lesions, aggregate volume, largest size lesion, and regions of the body involved. |
| Identification of any palpable lymph node, especially those ≥1.5 cm in largest diameter or firm, irregular, clustered, or fixed. |
| Identification of any organomegaly. |
| Skin biopsy |
| Most indurated area if only 1 biopsy. |
| Immunophenotyping to include at least the following markers: CD2, CD3, CD4, CD5, CD7, CD8, and a B-cell marker such as CD20. CD30 should be considered especially in cases where lymphomatoid papulosis, anaplastic lymphoma, or large-cell transformation is considered. CCR4 if mogamulizumab available. |
| Evaluation for clonality of TCR gene rearrangement. |
| Blood tests |
| CBC with manual differential, liver function tests, LDH, comprehensive chemistries. |
| TCR gene rearrangement and relatedness to any clone in skin. |
| Analysis for abnormal lymphocytes by either Sézary cell count with determination absolute number of Sézary cells and/or flow cytometry (including CD4+/CD7− or CD4+/CD26−). |
| Radiologic tests |
| In patients with T1N0B0 stage disease who are otherwise healthy and without complaints directed to a specific organ system, and in selected patients with T2N0B0 disease with limited skin involvement, radiologic studies may be limited to a chest radiograph or ultrasound of the peripheral nodal groups to corroborate absence of adenopathy. |
| In all patients with other than presumed stage IA disease, or selected patients with limited T2 disease and the absence of adenopathy or blood involvement, CT scans of chest, abdomen, and pelvis alone ± FDG-PET scan are recommended to further evaluate any potential lymphadenopathy, visceral involvement, or abnormal laboratory tests. In patients unable to safely undergo CT scans, MRI may be substituted. |
| Lymph node biopsy |
| Excisional biopsy is indicated in those patients with a node that is either ≥1.5 cm in diameter and/or is firm, irregular, clustered, or fixed. |
| Site of biopsy: Preference is given to the largest lymph node draining an involved area of the skin or if FDG-PET scan data are available, the node with highest SUV. If there is no additional imaging information and multiple nodes are enlarged and otherwise equal in size or consistency, the order of preference is cervical, axillary, and inguinal areas. |
| Analysis: pathologic assessment by light microscopy, flow cytometry, and TCR gene rearrangement. |
| Recommended evaluation/initial staging . |
|---|
| Complete physical examination including |
| Determination of type(s) of skin lesions. |
| If only patch/plaque disease or erythroderma, then estimate percentage of BSA involved and note any ulceration of lesions. |
| If tumors are present, determine total number of lesions, aggregate volume, largest size lesion, and regions of the body involved. |
| Identification of any palpable lymph node, especially those ≥1.5 cm in largest diameter or firm, irregular, clustered, or fixed. |
| Identification of any organomegaly. |
| Skin biopsy |
| Most indurated area if only 1 biopsy. |
| Immunophenotyping to include at least the following markers: CD2, CD3, CD4, CD5, CD7, CD8, and a B-cell marker such as CD20. CD30 should be considered especially in cases where lymphomatoid papulosis, anaplastic lymphoma, or large-cell transformation is considered. CCR4 if mogamulizumab available. |
| Evaluation for clonality of TCR gene rearrangement. |
| Blood tests |
| CBC with manual differential, liver function tests, LDH, comprehensive chemistries. |
| TCR gene rearrangement and relatedness to any clone in skin. |
| Analysis for abnormal lymphocytes by either Sézary cell count with determination absolute number of Sézary cells and/or flow cytometry (including CD4+/CD7− or CD4+/CD26−). |
| Radiologic tests |
| In patients with T1N0B0 stage disease who are otherwise healthy and without complaints directed to a specific organ system, and in selected patients with T2N0B0 disease with limited skin involvement, radiologic studies may be limited to a chest radiograph or ultrasound of the peripheral nodal groups to corroborate absence of adenopathy. |
| In all patients with other than presumed stage IA disease, or selected patients with limited T2 disease and the absence of adenopathy or blood involvement, CT scans of chest, abdomen, and pelvis alone ± FDG-PET scan are recommended to further evaluate any potential lymphadenopathy, visceral involvement, or abnormal laboratory tests. In patients unable to safely undergo CT scans, MRI may be substituted. |
| Lymph node biopsy |
| Excisional biopsy is indicated in those patients with a node that is either ≥1.5 cm in diameter and/or is firm, irregular, clustered, or fixed. |
| Site of biopsy: Preference is given to the largest lymph node draining an involved area of the skin or if FDG-PET scan data are available, the node with highest SUV. If there is no additional imaging information and multiple nodes are enlarged and otherwise equal in size or consistency, the order of preference is cervical, axillary, and inguinal areas. |
| Analysis: pathologic assessment by light microscopy, flow cytometry, and TCR gene rearrangement. |
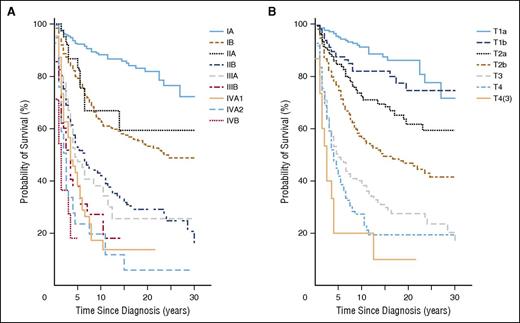
In contrast, the prognosis for patients presenting with advanced-stage disease is poor (Table 6; Figure 1).11,12,14,15 A variety of potential prognostic factors have been identified and 2 separate prognostic models for early- and advanced-stage disease have been proposed based on multivariate analysis identifying independent poor risk factors; a Cutaneous Lymphoma International Consortium (CLIC) has established a prospective study to establish a prognostic index (Cutaneous Lymphoma International Prognostic Index).13,15-24
Prognosis in MF/SS
| Stage . | 5-y OS, % . | 10-y OS, % . |
|---|---|---|
| IA | 94-100 | 88 |
| T1a | 97 | 91 |
| T1b | 91 | 80 |
| IB | 73-86 | 70 |
| T2a | 85 | 75 |
| T2b | 81 | 64 |
| IIA | 78 | 52 |
| IIB | 40-65 | 34 |
| IIIA | 47-60 | 37 |
| IIIB | 40-56 | 25 |
| IVA1 | 37-48 | 18 |
| IVA2 | 18-33 | 15 |
| IVB | 18-39 | NR |
| Stage . | 5-y OS, % . | 10-y OS, % . |
|---|---|---|
| IA | 94-100 | 88 |
| T1a | 97 | 91 |
| T1b | 91 | 80 |
| IB | 73-86 | 70 |
| T2a | 85 | 75 |
| T2b | 81 | 64 |
| IIA | 78 | 52 |
| IIB | 40-65 | 34 |
| IIIA | 47-60 | 37 |
| IIIB | 40-56 | 25 |
| IVA1 | 37-48 | 18 |
| IVA2 | 18-33 | 15 |
| IVB | 18-39 | NR |
NR, not reached.
Disease-specific survival (MF/SS). According to clinical (A) and skin stage (B) (Agar et al23 ).
Disease-specific survival (MF/SS). According to clinical (A) and skin stage (B) (Agar et al23 ).
Clinical case 1. Early-stage IB-IIA intermediate-risk disease
Scenario
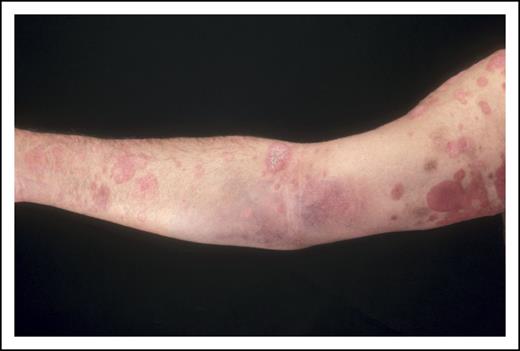
A 62-year-old man presented with a 5-year history of patches and plaques having failed topical and systemic treatment of psoriasis (Figure 2). Examination revealed polymorphic patches and plaques with involvement of limb girdle sites involving 45% of the body surface area (BSA) with a modified severity weighted assessment (mSWAT) score of 60 (percentage of patches = 30 × 1 + percentage of plaques = 15 × 2 giving a total mSWAT of 60: see Cutaneous Lymphoma Resource Tools app; Guys and St Thomas' NHS Foundation and Cranworth Medical Ltd). There was no peripheral lymphadenopathy, and blood tests including lymphocyte subsets were normal. Human T-cell lymphotropic virus type 1 was negative. A biopsy from a patch showed a lymphoid infiltrate (CD3+CD4+CD7−CD30− with a low proliferation index) and subtle exocytosis of lymphocytes into the overlying epidermis. Biopsy of a plaque revealed a folliculotropic infiltrate with medium-sized lymphoid cells showing mild cytologic atypia and an identical phenotype but no evidence of large-cell transformation (LCT). A discrete T-cell clone was detected in the plaque but not from the patch or peripheral blood. These clinicopathologic features were consistent with MF (folliculotropic variant stage IB; T2b B0a).
Comment
This presentation is typical of many patients with early-stage disease. However, the presence of folliculotropic plaques (T2b) can be associated with a higher risk of disease progression.23 The presence of plaques alone (T1b/T2b) in early-stage patients also indicates a poorer prognosis, presumably reflecting a larger tumor burden or possibly greater resistance to skin-directed treatments. Utilizing a proposed prognostic index, this patient has at least 2 adverse factors suggesting an intermediate prognosis with an expected 5-year survival of 87.5%.24
Management
The patient commenced psoralen with UVA (PUVA) phototherapy 2 times weekly for 16 weeks with a partial response (PR) although persistent plaques were present in flexural sites (sanctuary sites: mSWAT = 9). PUVA was continued for a further 3 months weekly but with little additional benefit. Cutaneous disease within the sanctuary sites was treated with localized superficial radiotherapy (8 Gy in 2 fractions) with complete resolution. However, within 8 months, the patient had a relapse of extensive patches and plaques (mSWAT = 45). In view of the short duration of response with PUVA, alternative options including bexarotene or α interferon (IFN-α), or total skin electron beam (TSEB) therapy, were considered. The patient elected to receive low-dose TSEB (12 Gy in 8 fractions). This produced a complete response (CR) and was well tolerated. The patient remained disease-free for 15 months but then relapsed with extensive cutaneous patches and plaques (mSWAT = 50). Palpable peripheral inguinal nodes were noted on examination and 18F-flurodeoxyglucose (FDG) positron emission tomography (PET)–computed tomography (CT) scanning demonstrated low-grade uptake within the inguinal and femoral nodes (maximum standardized uptake value [SUVmax] = 3) with the largest right inguinal node measuring 2 cm in the longest diameter. No other abnormality was noted. An excisional biopsy of the right inguinal node showed dermatopathic features (lymph node stage N1; overall stage IIA). The patient was treated with IFN-α 3 million units (MU) 3 times per week with an excellent PR after 3 months. The patient remains on maintenance IFN-α with a sustained PR for the past 20 months. If the patient develops extensive cutaneous relapse or progression, we will consider retreatment with PUVA (in order to achieve a rapid response) and oral bexarotene as a maintenance therapy. Alternatively, the patient could receive another course of low-dose TSEB. Unlike high-dose TSEB (typically 30-36 Gy), low-dose regimens can be repeated as needed. In addition, the patient would be a candidate for clinical trials.
Comment
This patient’s progress highlights the frequent ongoing need for multiple skin-directed and systemic treatment interventions for patients with extensive cutaneous disease (T2b). Maintenance systemic treatment may also be required in patients with early-stage disease who lack a durable response to skin-directed treatment. To date, there have been no well-designed comparative studies of PUVA and UVB but there is a consensus that narrowband UVB (TLO1) has a lower response rate and duration of remission than PUVA, especially for thicker plaques.25,26 Response rates to PUVA phototherapy in patients with patches only (T1a/T2a) are high, with CR rates of ∼30% to 58% and overall response rates (ORRs) of 70% to 95%.27,28 However, although PUVA phototherapy is effective for the majority of patients, durable complete responses are rare and the total lifetime cumulative UVA dose must not exceed 1000 J/cm2 or 250 sessions because of an increased rate of skin malignancies including squamous cell carcinoma.29-31 In addition, maintenance PUVA treatment rarely seems to add significant value. On the other hand, UVB phototherapy (TLO1) is more convenient, has a better safety profile than PUVA, and is widely available. We often initiate UVB (TLO1) for patients with predominant patches, switching to PUVA if there is a suboptimal response or alternatively start with PUVA for patients with extensive thick plaques. Although topical therapies including corticosteroids have minimal benefit for patients with extensive skin disease, mechlorethamine (nitrogen mustard) can be an effective alternative or complimentary skin-directed treatment option. Mechlorethamine gel (0.02%) has similar efficacy to compounded preparations and is now approved by the US Food and Drug Administration (FDA) but is not widely available outside of the United States.32
The combination of PUVA and IFN-α or bexarotene has been studied in the context of prospective phase 3 trials.33-35 The EORTC 21011 study indicated a CR rate of 27% for PUVA which is lower than reported in uncontrolled studies.36 These studies were not powered to identify whether the UV dose was lower for the combination therapies but there appears to be a trend. However, overall response rates (70%) appear similar in both studies for PUVA alone compared with the combination with either IFN-α or bexarotene. These trials did not clarify the role of maintenance IFN-α or bexarotene after PUVA but this is an option.37-40
Both bexarotene and IFN-α monotherapy achieve response rates (RRs) of about 30%, but complete remissions are rare.38 IFN-α should only be considered as a second-line therapy for early-stage disease and is effective at doses of 3 to 9 MU 3 to 7 times per week.41-43 It is not clear if pegylated IFN-α has a better efficacy but the once-weekly schedule is more convenient. Time to response is in the order of weeks and it can be combined with other modalities.33,34,39,40,44-47 Low-dose methotrexate (MTX) can be effective for patients with early stages of MF whose disease is resistant to skin-directed therapies. The largest series included 69 patients with patch/plaque MF (T2b) treated with doses up to 75 mg weekly (median 25 mg once weekly), achieving a 12% CR and 22% PR rate with a median time to treatment failure of 15 months.48 Low-dose MTX has also been successfully combined with IFN-α.49
Cutaneous lymphomas are highly radiosensitive and radiotherapy is a key palliative therapy for MF especially for sanctuary sites resistant to phototherapy.50 Partial regression of disease may be observed with single doses as low as 1.0 Gy51 and single doses as low as 8 Gy achieve a CR rate of 94.4%.52 For most patients, the target volume is the epidermis and/or dermis, unless there are tumors or deep ulcers. Most lesions may therefore be treated with soft (low penetrance) beam–superficial radiograph therapy (50-145 peak kilovoltage) or 4-9 MeV electron beams. Higher energy beams (electrons or orthovoltage/megavoltage radiographs) are occasionally necessary for thicker lesions. Treatment with low-dose regimens such as 8 to 12 Gy in 2 to 4 fractions is intended to improve symptoms. Rarely, patients present with truly localized MF (unilesional). Our approach is to treat such patients with local radiotherapy with “curative” intent to a dose of ∼30 Gy. A large proportion of these patients may remain disease-free.53
TSEB therapy remains an excellent treatment option for MF. Its high rate of CR leads to a significant clinical benefit but overall survival (OS) may not be affected.54,55 TSEB therapy requires the use of either multiple field arrangements or a rotational technique, with “patching” or “boosting” for areas of underdosing and self-shielding (eg, soles of feet, perineum) and the full dose regimen takes 6 to 10 weeks to complete. The likelihood of achieving a CR and durability of response decreases with increasing stage of disease; patients with T1 disease have a >80% CR rate with radiotherapy (either local field or TSEB therapy), compared with 20% to 30% CR rates for T4 disease. Five-year relapse-free survival rates with radiation alone are 40% to 60% for T1 disease, but <10% for T4 disease.53
Historically, TSEB was delivered at high doses (30-36 Gy in 20-36 fractions), which is the limit of skin tolerance for a single course of treatment. Recent data from several centers indicate that low-dose regimens (10-12 Gy in 8-12 fractions) can produce excellent overall RR of 88% to 95%. The CR rates (27%-57%) are lower than those achieved with high-dose therapy, but the duration of response (6-15 months) is similar to TSEB with doses of 30 Gy or greater.56,57 Despite the lower CR rate associated with low-dose TSEB, this approach is justifiable because repeat courses are well tolerated and can provide excellent palliation. TSEB therapy may be followed by maintenance PUVA, topical nitrogen mustard, and other options such as bexarotene or IFN-α.54,58,59
Case 2. Erythrodermic disease
Scenario
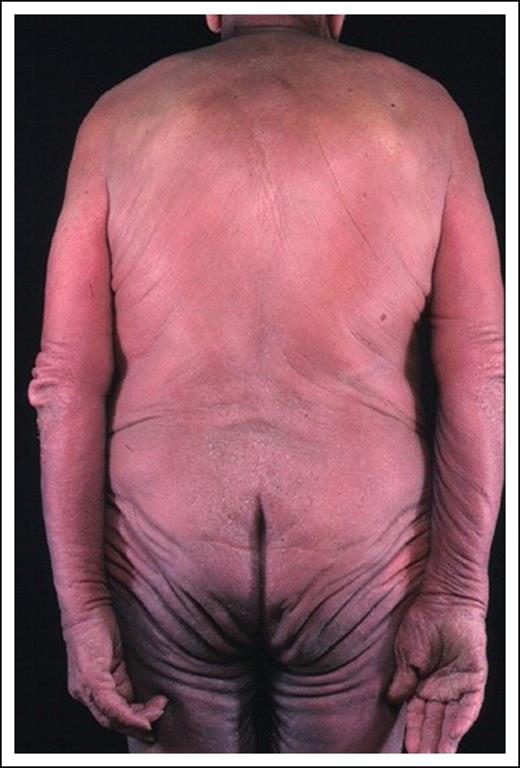
A 60-year-old man presented with a 4-year history of generalized erythema and marked scaling with intense pruritus (mSWAT = 140 based on percentage of patches of 40 × 1 + percentage of plaques of 50 × 2, resulting in total 90% skin involvement with 50% of the skin demonstrating either edema, fissuring, or lichenification) (Figure 3). Peripheral inguinal and axillary nodes were palpable and a PET-CT scan confirmed that the largest axillary node measured 2.5 cm with moderate FDG avidity (SUVmax, 3.8). A node biopsy revealed only dermatopathic change. A skin biopsy showed an upper dermal lymphohistiocytic T-cell infiltrate with no antigen loss and minimal cytologic atypia. A complete blood count (CBC) revealed a peripheral blood lymphocytosis (PBL; 5 × 109/L), and flow cytometry revealed a CD4+ lymphocytosis (4.5 × 109/L) with a relatively low CD8 count (1.50 × 109/L), an expanded CD4+CD26− (50%) and CD4+CD7− (60%) population, and abnormal mononuclear cells on a peripheral blood smear consistent with Sézary cells. These findings were consistent with SS (stage IVA1).
Management
The patient was treated with bexarotene (300 mg daily with concomitant rosuvastatin 40 mg daily and levothyroxine 150 μg daily) and extracorporeal photopheresis (ECP) monthly. After 6 months, a significant improvement in erythema was noted (mSWAT decreased from 140 to 75) with reduction in severity of pruritus. The PBL also improved (3.5 × 109/L). A PR was sustained for a further 4 months but the patient then developed a recurrence of erythroderma (mSWAT = 130) and the PBL increased to 7 × 109/L. Repeat PET-CT scan showed FDG-avid bulky axillary nodes (SUVmax, 8) and a core biopsy demonstrated effacement with LCT (CD4+CD30−). Alemtuzumab (10 mg subcutaneous 3 times weekly for 12 weeks) was commenced and produced a clinical CR in skin, nodes, and blood. At this point, consolidation with a reduced-intensity allogeneic transplant was discussed but the patient had no siblings and so an unrelated donor search was initiated.
Comment
This case illustrates a number of key issues. The diagnosis of erythrodermic disease can be very challenging as skin histology may be nondiagnostic in 30% of cases but in this case the presence of a peripheral blood CD4 lymphocytosis and identical T-cell clones in skin, blood, and node supported a diagnosis of SS.60 The flow results (CD4+CD26− >30% and CD4+CD7− >40%) and proportion of Sézary cells (>1.0 × 109/L) confirm that the patient has SS (blood stage B2; clinical stage IVA1) as opposed to E-MF with no blood involvement (stage IIIA) or low levels of Sézary cells (>5% of PBL but total Sézary count <1.0 × 109/L: stage IIIB). The Sézary cell morphology can be subtle unless patients have the large-cell variant and flow results can be challenging as there are no specific markers of Sézary cells. Results of an EORTC multicenter study to refine the SS phenotype are awaited.
Prognosis in SS is dependent on absence (stage IVA1) or presence (stage IVA2) of nodal involvement with OS 37% and disease-specific survival 41% at 5 years for stage IVA1. The median survival for SS is 3.1 years with an OS of 26% at 5 years compared with E-MF without blood involvement (stage IIIA: median survival, 4.7 years; OS, 47%-58% at 5 years). However, the prognosis for erythrodermic SS/MF patients can be further stratified using a proposed prognostic index with overall median survival from 1.5 to 10.2 years depending on 3 independent adverse factors: patient age, presence of lymph node disease, and peripheral blood involvement.23,60,61 In multivariate analysis, advanced age and elevated lactate dehydrogenase (LDH) are the strongest predictors of a poor prognosis.62 However, the development of skin tumors in erythrodermic SS or E-MF patients is associated with a dismal prognosis.23
There are no randomized clinical trial data in SS but there is a consensus (NCCN and EORTC) that chemotherapy options are not appropriate first-line options (Figure 4). In contrast, an immunobiologic approach consisting of either ECP alone or combined with IFN-α and bexarotene (reviews63 ) can provide clinical responses in 30% to 80%64 (Table 2). However, the presence of high-grade transformed nodal disease is less likely to respond to triple therapy. Second-line options include alemtuzumab which appears to be more effective in erythrodermic MF/SS than advanced stages of MF without peripheral blood involvement. Phase 2 studies using standard and low-dose alemtuzumab have shown ORR of 75% and a median duration of response of 12 months despite risks of CMV reactivation and hematological toxicity.65-70
Recent FDA approval of the histone deacetylase inhibitors (HDACi), vorinostat (suberoylanilide hydroxamic acid) and romidepsin (depsipeptide), for relapsed/refractory cutaneous T-cell lymphoma (CTCL) provides an alternative second-line option for SS. Vorinostat, an orally available hydroxamic acid derivative, inhibits both class I and II histone deacetylases and open-labeled studies reported ORR of 24% to 30%, with a reduction in pruritus in 58% of patients.71,72 The most common toxicities are gastrointestinal or constitutional symptoms, hematologic abnormalities, and taste disorders, and are usually of mild to moderate severity.73 Romidepsin has shown higher RRs (38%) with a subset of patients achieving durable remissions and there is evidence that HDACi may be more effective in E-MF and SS.74-77 The global scoring system in the romidepsin study has since been refined by the ISCL and is now a standard for clinical trials.78 Recent phase 2 studies of anti-CCR4 (mogamulizumab) have shown excellent responses in relapsed/refractory SS (47%).79
Single-agent chemotherapy regimens, including liposomal doxorubicin and gemcitabine, can be effective but the duration of response is short.80 Reasonable palliation in E-MF and SS can also be achieved with single-agent low-dose oral methotrexate and chlorambucil. Although radiotherapy and TSEB is rarely effective in erythrodermic disease, radiotherapy can be used with palliative intent for local disease control when erythrodermic patients develop skin tumors, localized bulky peripheral nodal disease, or oropharyngeal disease.
Transplantation
Interpretation of transplant data is limited because the number of patients with MF/SS treated to date is small. The European Society for Blood and Marrow Transplantation (EBMT) and the Center for the International Blood and Marrow Transplant (CIBMTR) have recently published their registry data.81-83 In brief, standard-intensity allogeneic stem cell transplantation can induce complete and durable remissions in patients with CTCL. Although infection rates, early relapse, and nonrelapsed mortality were relatively high, durable CRs are observed84-86 and outcomes following reduced-intensity regimens have been reported on over 100 patients with advanced stages of MF and SS.83 There is some evidence emerging that SS patients have better outcomes whereas those with stage IVB may be poor candidates.82,87,88 We believe the role of allogeneic transplantation in patients with advanced-stage MF and SS should be considered as consolidation following achievement of partial or complete remissions.
Clinical case 3. Advanced disease (MF stages IIB-IVB)
Scenario
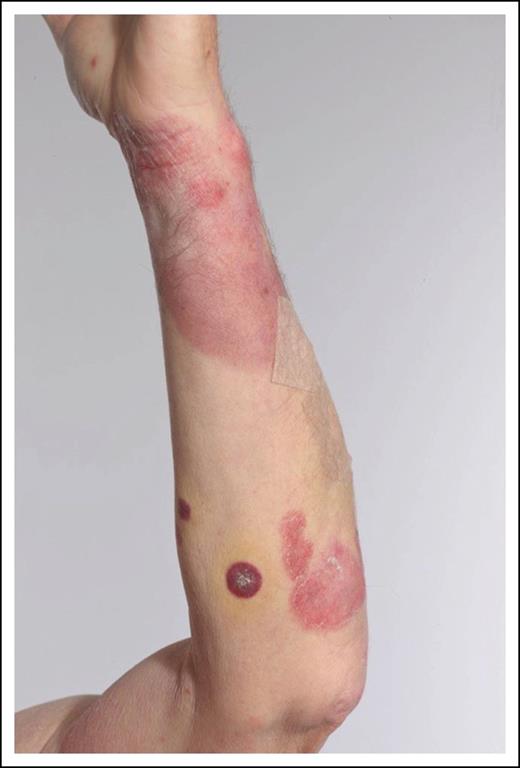
A 52-year-old woman presented with an 18-month history of polymorphic patches and plaques involving the limbs (Figure 5) and pelvic girdle area. A diagnosis of MF was made on the basis of diagnostic clinicopathologic features (stage IB/T2bN0M0B0b).
Management
Phototherapy was successful but the patient developed an ulcerated 4-cm tumor (stage IIB/T3) on the right wrist and a biopsy showed a nonepidermotropic infiltrate consisting of medium-sized pleomorphic T cells (CD3+CD4+CD30+). A staging PET-CT scan was normal apart from PET avid uptake within the skin tumor (SUVmax = 7). The tumor responded to radiotherapy and PUVA was continued with an excellent PR, allowing withdrawal of therapy after 5 months. The patient remained well for 4 years with limited skin disease treated successfully with a further course of PUVA and local radiotherapy for 2 small- to medium-sized tumors. However, the development of a bulky axillary node (3 cm) was noted and a core biopsy showed partial effacement with large atypical blast-like lymphocytes (CD4+CD30+) consistent with LCT (stage IVA2). A PET-CT scan showed no other abnormality and the patient was treated with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) chemotherapy. An initial CR after 2 cycles was followed by a recurrence of extensive cutaneous disease (characterized by patches/plaques and scattered tumors) after 4 cycles despite complete resolution of nodal disease.
The patient lost weight and complained of difficulty swallowing. A PET-CT scan revealed an FDG-avid mass (SUVmax, 14) within the oropharynx and several small but FDG-avid masses (SUVmax, 8) within the lower lobes of both lungs (stage IVB). A biopsy of the oropharyngeal mass revealed transformed high-grade lymphoma (CD3+CD4−CD30+) with an identical T-cell clone to that in skin and node. The oropharyngeal mass resolved after radiotherapy. Options considered were palliative single-agent chemotherapy (gemcitabine) or a clinical trial assessing the use of brentuximab vedotin in chemotherapy-refractory advanced-stage MF. The patient entered the clinical trial and was randomized to brentuximab vedotin. The pulmonary nodules and the skin tumors resolved after only 4 cycles although there was only a partial resolution of cutaneous patches and plaques. The patient continued on brentuximab vedotin and a donor search for a reduced-intensity conditioning allogeneic transplant was initiated.
Comment
Although most patients with early-stage disease have an indolent course, progression can occur in 24% of patients.23 CD30 expression can occur in the absence of LCT in MF and such patients may have an indolent clinical course.15,89 In addition, CD30 expression does not indicate a primary cutaneous anaplastic large-cell lymphoma when the patient has a preceding history of MF.
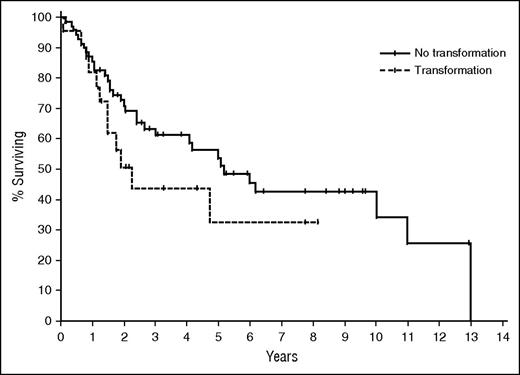
The role of PET imaging in MF and other CTCL variants remains unclear but FDG-avid disease in skin is readily detected and PET can identify the most appropriate peripheral node to be biopsied.90 This patient developed LCT at the time of node biopsy. LCT is currently defined as the presence of large cells (≥4 times the size of a small lymphocyte) in >25% of the infiltrate, or if these cells form microscopic nodules.91,92 There is a variable reported incidence of transformation in MF of 8% to 39% and the prognostic relevance of LCT is unclear with studies suggesting a poor prognosis of <2 years (Figure 6) or that LCT is only predictive of progression risk.15,16,23,93 Although transformation is more often associated with advanced stage,15,91 LCT can also occur in early-stage disease.
Time to next treatment: IVA-IVB disease. OS of patients with advanced-stage disease according to presence (n = 22) or absence (n = 70) of LCT in the advanced-stage population.15 Median OS in the transformed group was 2.2 years, compared with 5.2 years in the nontransformed group. TTNT, time to next treatment.
Time to next treatment: IVA-IVB disease. OS of patients with advanced-stage disease according to presence (n = 22) or absence (n = 70) of LCT in the advanced-stage population.15 Median OS in the transformed group was 2.2 years, compared with 5.2 years in the nontransformed group. TTNT, time to next treatment.
This case also emphasizes the clinical heterogeneity of MF and the challenge of deciding when to escalate therapy as some patients with advanced but limited disease (stage IIB) can be successfully managed with skin directed therapy including radiotherapy, whereas patients with extensive skin tumors (stage IIB) or nodal disease (stage IVA2) invariably require aggressive therapies including chemotherapy, although radiation therapy remains an important palliative approach.
Although systemic multiagent chemotherapy is often considered in patients with advanced-stage disease, the original randomized National Cancer Institute (NCI) study in MF demonstrated that combination chemoradiotherapy offered no survival benefit over “conservative” sequential therapy.94 Moreover, relatively rapid relapses are observed following chemotherapy.80 Critically following chemotherapy, patients will often have resistant or relapsed disease characterized by only cutaneous patches and plaques which will require skin-directed therapy rather than a traditional escalation of systemic therapy. The choice of systemic therapy therefore depends largely on age, performance status of patient, tempo of the disease, and risks of myelosuppression as well as stage.
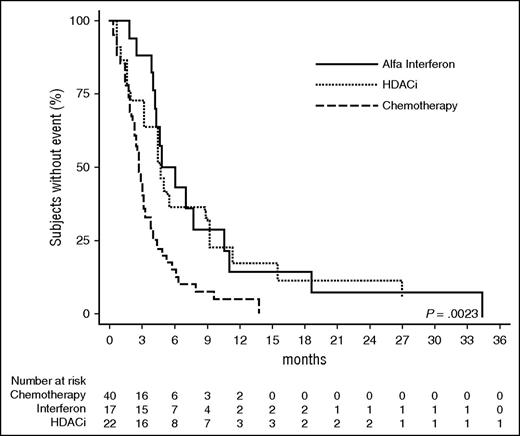
A number of chemotherapy agents have demonstrated activity in MF/SS95 (review80 ). In brief, systemic agents include alkylating agents (cyclophosphamide, chlorambucil), anthracyclines, purine analogs, and etoposide. Although single-agent or combination chemotherapy regimens have produced moderately high response rates in patients with advanced-stage MF/SS, these responses are invariably not durable. There is no recognized superior multiagent chemotherapy regimen for MF, and CHOP-based regimens have a disappointing track record.80 Because of the high risk of infection and myelosuppression as well as modest response durations with combination chemotherapy, single-agent therapies are generally preferred except in younger patients who are refractory or who present with extensive adenopathy and/or visceral involvement or constitutional symptoms and require rapid tumor reduction. Thus, in patients with relatively slowly progressive disease who have failed other treatments, we would consider chlorambucil, cyclophosphamide, or etoposide, and for patients with more rapidly progressive disease, single-agent gemcitabine,96-99 pentostatin,45,100-105 or liposomal doxorubicin106-108 as these agents have shown ORRs of 50% to 80%. However, the EORTC 21012 study of liposomal doxorubicin showed lower responses (ORR 40% and 6% CR).109 Gemcitabine has a high RR but myelosuppression can be problematic.99 A phase 2 study assessing the role of combination gemcitabine and bexarotene showed no significant benefit in terms of progression-free survival (5.3 months) with ORR of only 31%.110 Given the overall poor RRs and brief durations of response (Figure 7), chemotherapy mostly remains a palliative option and a better strategy may be to use biologic therapies such as interferon or HDACi (Figure 7).80
OS: LCT. Time to next treatment (MF/SS) comparing chemotherapy with HDACi and IFN-α (Hughes et al80 ).
OS: LCT. Time to next treatment (MF/SS) comparing chemotherapy with HDACi and IFN-α (Hughes et al80 ).
In phase 2 trials, brentuximab vedotin was associated with RRs in ∼70% of patients.111,112 Denileukin diftitox (DD) targets the IL-2 receptor subunit (CD25) and was approved after a placebo-controlled randomized trial of systemic therapy in MF/SS113 demonstrated a superior RR of 49.1% compared with placebo. Other systemic treatment options for such patients include HDACi such as vorinostat or romidepsin,74,75 pralatrexate,114 lenalidomide,115 or proteasome inhibitors.116,117 Finally, patients should be considered for novel agents in the context of clinical trials. Such new agents include, but are not restricted to, the monoclonal antibodies mogamulizumab (anti-CCR4),79 brentuximab vedotin (anti-CD30),111,112 and IPH4102 (anti-KIR3DL2).118
Summary
Although progress has been made in terms of prognostic stratification, the treatment of patients with advanced stages of MF and SS remains very challenging. MF and SS are usually resistant to chemotherapy and, although clinical responses to a wide variety of biologic agents are seen in 30% to 40% of patients, complete and durable responses are rare. This therapeutic challenge is exemplified by recent high-throughput sequencing studies in both SS and MF showing marked genomic heterogeneity affecting a variety of signaling pathways which suggests that the identification of appropriate small-molecule inhibitors for targeted therapy will also be challenging.119,120 In contrast, allogeneic transplantation has produced durable complete clinical remissions in a small number of patients but overall responses following transplant are still poor. Nevertheless, these remissions suggest that targeted immunotherapy options should be considered.
Authorship
Contribution: S.W., R.H., and H.M.P. agreed upon the design of the article, contributed to the written text, and edited/reviewed/approved the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sean Whittaker, St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust and Division of Genetics and Molecular Medicine, King’s College London, London SE1 9RT, United Kingdom; e-mail: sean.whittaker@kcl.ac.uk.