Abstract
Left ventricular assist devices (LVAD) provide cardiac support for patients with end-stage heart disease as either bridge or destination therapy, and have significantly improved the survival of these patients. Whereas earlier models were designed to mimic the human heart by producing a pulsatile flow in parallel with the patient’s heart, newer devices, which are smaller and more durable, provide continuous blood flow along an axial path using an internal rotor in the blood. However, device-related hemostatic complications remain common and have negatively affected patients’ recovery and quality of life. In most patients, the von Willebrand factor (VWF) rapidly loses large multimers and binds poorly to platelets and subendothelial collagen upon LVAD implantation, leading to the term acquired von Willebrand syndrome (AVWS). These changes in VWF structure and adhesive activity recover quickly upon LVAD explantation and are not observed in patients with heart transplant. The VWF defects are believed to be caused by excessive cleavage of large VWF multimers by the metalloprotease ADAMTS-13 in an LVAD-driven circulation. However, evidence that this mechanism could be the primary cause for the loss of large VWF multimers and LVAD-associated bleeding remains circumstantial. This review discusses changes in VWF reactivity found in patients on LVAD support. It specifically focuses on impacts of LVAD-related mechanical stress on VWF structural stability and adhesive reactivity in exploring multiple causes of AVWS and LVAD-associated hemostatic complications.
A brief history and clinical applications of LVAD
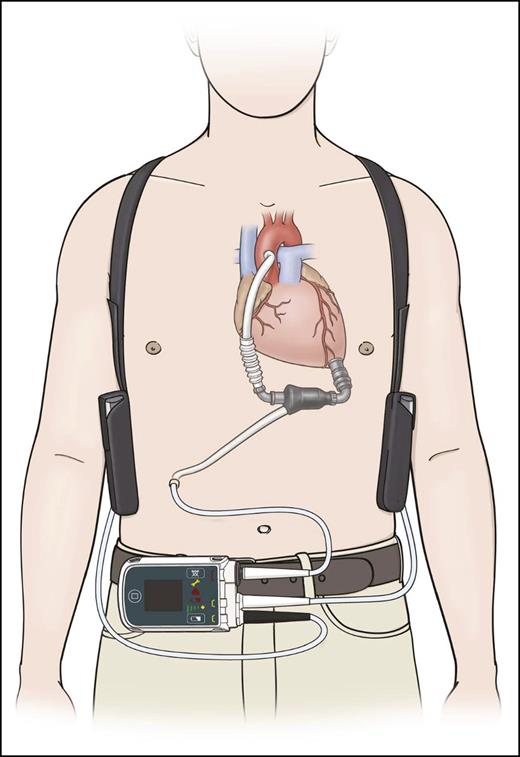
The first ventricular assist device (VAD) was implanted by Michael E. Debakey in 1966 at the Methodist Hospital in Houston, Texas. The interest in developing a device for artificially supporting the circulation was further increased by the first total artificial heart implantation, performed by Denton A. Cooley in Houston on April 4, 1969.1 In 1986, O. H. Frazier and his colleagues began implanting the first generation of pneumatic pulsatile LVADs (the Heartmate 1000 IP) to improve end-organ perfusion in candidates for heart transplant.2 The patients with LVAD implants experienced significant improvements in long-term survival. The first continuous-flow LVAD—the Hemopump—was subsequently implanted in 1988.3 It was followed by successful implantations of the HeartMate XVE in 1991,4 the Jarvick 2000 in 2000,5 and the HeartMate2 (Figure 1) in 2003 by O. H. Frazier and his colleagues at St. Luke’s Hospital in Houston, Texas.6 Continuous-flow LVADs are designed to provide blood flow of up to 10 L/min by generating a continuous blood flow from the inlet through the revolving impeller to the outlet. They energize blood flow throughout the cardiac cycle, thereby activating the normally passive flow of diastole. This continuous and unidirectional flow allows for a simplified device design and a smaller size, because it obviates the need for unidirectional valves.
Schematic illustration of an implanted continuous-flow HeartMate2 LVAD (with permission from Thoratec Corporation).
Schematic illustration of an implanted continuous-flow HeartMate2 LVAD (with permission from Thoratec Corporation).
The axial continuous-flow HeartMate2 is currently the device approved by the U.S. Food and Drug Administration (FDA) for both bridge-to-transplant (BTT)7 and destination therapy (DT).8 It has been implanted in >15 000 patients worldwide.9 The centrifugal continuous-flow Heartware HVAD is an intrapericardial pump with an impeller suspended by a passive magnetic and hydrodynamic bearing that produces contact-free rotation (FDA approved for BTT10 ).
Heart failure is a severe medical condition affecting more than 5 million individuals and it is a leading cause of death in the US.11 Cardiac transplantation remains a valid option in the treatment of these patients. However, only 2000 to 2500 transplants are performed each year because of limited organ availability. More than 4000 patients are currently on the waiting list (www.unos.org/data/transplant-trends/#waitlists_by_organ), and this number is expected to grow substantially because of our increasingly aging population. Patients on the waiting list have a significantly higher rate of mortality than those who receive the implants. LVADs were introduced as a rescue strategy for patients dying while on the heart transplant waiting list. They were used as a temporary solution until a donor heart could be transplanted (BTT).2 The early positive results of BTT led the FDA to approve the use of LVADs as an alternative to heart transplants, serving as a destination therapy.12 In some cases, LVADs have been shown to restore sufficient cardiac function and end-organ perfusion that patients who were previously ineligible for heart transplants eventually met transplantation criteria (bridge to decision). In rare cases, patients have had their LVADs successfully explanted (bridge to recovery).13,14 All surgical interventions alter internal anatomy to a condition that is not normal, but is better than the diseased condition it corrects. In the case of LVAD, the alteration is to the blood flow. Although patients have survived with normal activity for >10 years without a pulse, a LVAD can result in complications in some patients related to the unique levels and patterns of blood flow created by the device.
LVAD-related hemostatic complications
Major LVAD-associated complications include bleeding, neurologic dysfunction (transient ischemic attack and stroke), pump thrombosis, infection, right ventricular failure, and aortic regurgitation as codified by the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS).15 The registry collects clinical data on mechanical circulatory support devices from index hospitalization through follow-up evaluation on 16 268 patients from 163 centers (as of August 2015).
Neurologic dysfunction, pump thrombosis, and bleeding are common hemostatic complications. A stroke rate of 0.064 event per patient per year (EPPY) was reported in a 7.5-year study of 230 patients implanted with the HeartMate2 as either BTT (80.4%) or DT (19.6%),16 significantly higher than the 0.013 to 0.035 EPPY reported for patients with advanced heart failure.8,17 The stroke rates for the Heartware were 0.11 and 0.09 EPPY for ischemic and hemorrhagic stroke, respectively.10 LVAD-supported patients who experienced stroke had twice the risk of death as stroke-free patients.16
Pump thrombosis is usually located at the bearing of the device and presents at inspection as a chronic fibrous pannus formation that responds poorly to thrombolytic therapy. Pump exchange is the treatment of choice.18 The incidence of pump thrombosis with the HeartMate2 was 2% to 4%, with 0.03 EPPY in the first year in a BTT trial7 ; and 0.024 EPPY over a 2-year period in a DT trial.8
LVAD-associated bleeding is the most frequent complication, occurring in 19% to 40% of patients on HeartMate2 support.19,20 INTERMACS defines LVAD-related bleeding as episodes of internal or external bleeding that result in death, reoperation, hospitalization, or transfusion of packed red blood cells (>4 U in the first 7 days, or any units >7 days after the implant). Bleeding within 7 days is primarily caused by surgery and the use of anticoagulants after the LVAD implantation. However, nonsurgical bleeding that occurs later has emerged as a major challenge to the recovery of patients on LVAD support. The mean time for nonsurgical bleeding and the rate of recurrent bleeding after LVAD implant varies significantly among published reports, ranging from 10 to 154 days,21-23 and 9.3% to 60%, respectively.22,24,25 Gastrointestinal (GI) bleeding and epistaxis are more common than mediastinum/thorax and intracranial bleeding.26-30 The incidence of GI bleeding increased significantly, from 0.068 EPPY for pulsatile LVADs to 0.63 EPPY for continuous-flow LVADs.31
Patients with clinical GI bleeding are treated in consultation with gastroenterologists, undergoing colonoscopy, upper endoscopic evaluation, and/or small bowel capsule endoscopy. In the presence of persistent GI bleeding with negative endoscopic findings, a tagged red blood scan or angiography is recommended. Optimal anticoagulation levels and an antiplatelet regimen for patients on LVAD support is an active area of research. In the absence of evident pump dysfunction, current guidelines32 recommend holding anticoagulation and antiplatelet therapy in the presence of clinically significant bleeding. During this holding period, strict clinical follow-ups are imperative because of the increased risk for device malfunction, pump thrombosis, and ischemic stroke.32,33 The US-TRACE study found that recurrent bleeding was reported in as much as 52% of cases despite the reduction in antithrombotic therapy, suggesting that anticoagulation might be only partially responsible for the hemorrhagic complications in these patients.33 Anticoagulation and antiplatelet therapy can be reintroduced with careful monitoring when the bleeding episode has resolved. In the case of refractory bleeding, the indication for warfarin and target international normalized ratio should be re-evaluated depending on the bleeding severity and pump types.32 Octreotide and thalidomide have been reported as off-label adjuvants for controlling recurrent GI bleeding,34-36 but neither has been systematically studied for the treatment of LVAD-related bleeding. Thalidomide is also associated with an increased risk of thrombosis.25,37
Attempts have been made to operate a continuous-flow device at a lower speed to reduce shear stress and to reintroduce pulsatility. At a lower LVAD speed, the native heart may increase (transiently and in systole only) the total cardiac output with minimal stroke volume. This approach has not been fully validated and may limit the amount of LVAD support to the native heart. New-generation devices have incorporated operational algorithms to introduce cardiac output variations over several cardiac cycles. These algorithms aim at simulating an arterial pulse pressure and, if effective, may reduce LVAD-induced bleeding. Additional information related to the clinical presentation and managements of LVAD-associated hemostatic defects is discussed by Susen et al in a recent review.38 In this review, we focus on the underlying mechanisms of LVAD-associated VWF abnormalities and their contributions to clinical bleeding.
GI bleeding is often found at the site of an arteriovenous malformation (AVM).39,40 There are at least 2 theories regarding how an AVM is formed. One is that high intraluminal pressure causes reflective and localized smooth muscle relaxation and arteriovenular dilation.39 The other is that intestinal hypoperfusion resulting from reduced pulse pressure leads to regional hypoxia, vascular dilation, and subsequent angiodysplasia.26 However, neither theory explains why the AVM is primarily formed in the GI track. Nevertheless, it was the association of AVM with GI bleeding that led to the hypothesis that LVAD-associated bleeding is similar to aortic valve stenosis (Heyde syndrome)41 in that it is caused by the deficiency of the adhesive ligand VWF and thus is often called acquired von Willebrand syndrome (AVWS).31,42,43
Biology of von Willebrand factor
VWF mediates platelet tethering to the subendothelial matrix at sites of vascular injury to initiate hemostasis. It also protects the coagulation factor VIII (FVIII) against proteolytic degradation by forming a VWF–FVIII complex in the circulation.44 As an acute-phase reactant, VWF synthesis and secretion is rapidly and significantly increased in response to inflammatory and ischemic insults. Elevated levels of VWF antigen and/or adhesion to platelets are, therefore, widely used as markers for endothelial perturbation and propensity for thrombosis and thromboembolism.
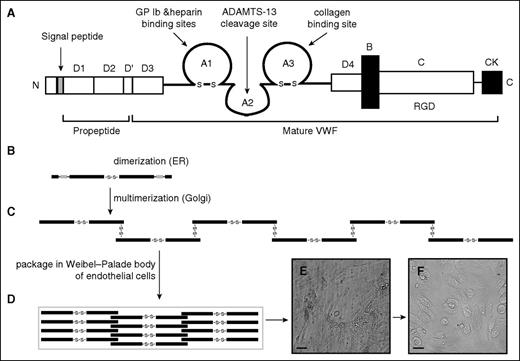
VWF is synthesized in megakaryocytes and endothelial cells as a single-chain propolypeptide of 2813 amino acids.44 A mature VWF monomer contains 4 types of repeated domains in the order of D1-D2-D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-CK (Figure 2A). The D′ and D3 domains mediate VWF multimerization and FVIII binding.45-48 The A1 and A3 domains contain the binding sites for the platelet glycoprotein (GP) Ib-IX-V complex and the subendothelial collagen, respectively. The A2 domain contains the Tyr1605-Met1606 peptide bond that is cleaved by a disintegrin and metalloprotease with thrombospondin type 1 repeat 13 (ADAMTS-13).49-51 The C domain contains an RGD sequence that interacts with integrins. Free thiols in the C domain are also involved in the shear-induced lateral association of VWF multimers to form highly adhesive fibrils.52,53
VWF domain structure and potential lateral association. (A) VWF domain structure. ProVWF forms disulfide-linked dimers (B) that further multimerize covalently to multimers in the Golgi (C). These multimers are packaged in the storage granule Weibel-Palade body of endothelial cells (D), where the multimerization process is likely to continue, but factors that regulate its rate and termination remain largely unknown. The stored VWF multimers are released from endothelial cells that are activated by inflammatory stimuli. These newly released VWF multimers form fibrillary meshes that capture platelets (E) and are proteolytically released from the surface of endothelial cells by ADAMTS-13 (F).
VWF domain structure and potential lateral association. (A) VWF domain structure. ProVWF forms disulfide-linked dimers (B) that further multimerize covalently to multimers in the Golgi (C). These multimers are packaged in the storage granule Weibel-Palade body of endothelial cells (D), where the multimerization process is likely to continue, but factors that regulate its rate and termination remain largely unknown. The stored VWF multimers are released from endothelial cells that are activated by inflammatory stimuli. These newly released VWF multimers form fibrillary meshes that capture platelets (E) and are proteolytically released from the surface of endothelial cells by ADAMTS-13 (F).
Once synthesized, pro-VWF monomers first dimerize through C-terminal disulfide bonds.54 A variable number of dimers then multimerize through the N-terminal disulfide bonds55-58 after the proteolytic removal of a 741-amino-acid propeptide (Figure 2B-C).54,59-62 The newly synthesized VWF multimers are either constitutively released or stored in the Weibel-Palade bodies (Figure 2D) of endothelial cells and the α-granules of megakaryocytes and platelets.63,64 VWF multimers stored in these granules are enriched in large and ultralarge (UL) forms that are released from activated endothelial cells.62,63,65-67 Newly released VWF multimers can be anchored to the surface of endothelial cells to form elongated and membrane-anchored VWF fibrils that are cleaved by ADAMTS-13 and released from the cells (Figure 2E).68,69 It is because of this unique structure that VWF adhesive activity is determined not only by antigen level, but also by the size of multimers, with large and UL multimers being most active in hemostasis and thrombosis/thromboembolism, respectively.56,70 This large and flexible structure also renders VWF prone to undergoing changes in conformation and adhesive activity in response to fluidic mechanical forces that are closely associated with LVAD implantation.
Regulation of VWF by physical forces
The most drastic change brought by a continuous LVAD is the conversion of a pulsatile flow of blood to a continuous flow with a significantly elevated shear rate and stress, not only in the device, but also systemically. An increase in fluid shear stress above certain thresholds is known to alter the structure of VWF multimers to induce VWF cleavage by ADAMTS-13 and also VWF binding to platelets (Figure 3).71-73
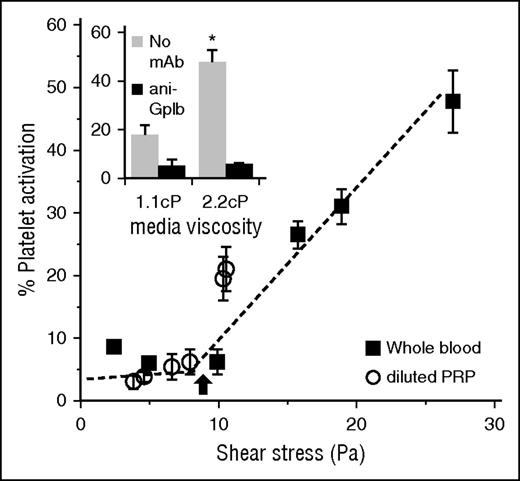
Shear-induced platelet activation. Platelets in diluted PRP or whole blood subjected to fluid shear in a cone-plate viscometer abruptly undergo activation above a critical wall shear stress of 8-10 Pa (arrow). Inset shows that this activation is a strong function of the mechanical force applied on the platelet GP Ibα by the VWF that is bound to it. Here, doubling media viscosity more than doubles the extent of cell activation (adapted from Shankaran et al71 ).
Shear-induced platelet activation. Platelets in diluted PRP or whole blood subjected to fluid shear in a cone-plate viscometer abruptly undergo activation above a critical wall shear stress of 8-10 Pa (arrow). Inset shows that this activation is a strong function of the mechanical force applied on the platelet GP Ibα by the VWF that is bound to it. Here, doubling media viscosity more than doubles the extent of cell activation (adapted from Shankaran et al71 ).
Among the continuous-flow devices, axial-flow LVADs have a high impeller speed that results in a high local shear rate and stress. Platelets in this device experience stresses of >10 Pa (1 Pa = 1 N/m2 = 10 dynes/cm2) during their passage, and some are exposed to stress as high as 600 Pa (Table 1).74-77 A centrifugal pump has a lower impeller velocity than an axial pump and lower wall shear stress throughout the device.76,77 Regions of high, nonphysiologic flow are formed in axial pumps with an estimated wall shear stress of >10 Pa, as is found in most of the devices.76,78 However, a recent study that compared the performance of centrifugal and axial-flow LVADs in 102 patients showed an equivalent loss of large VWF multimers and similar incidences of bleeding and thromboembolic events.79
Typical range of wall shear rates and stresses in blood vessels
| Blood vessel . | Shear rate (/s) . | Shear stress (Pa) . |
|---|---|---|
| Large arteries* | 300-800 | 1.4-3.6 |
| Arterioles* | 450-1600 | 2-7.2 |
| Veins* | 15-200 | 0.07-0.9 |
| Stenotic vessels* | 800-10 000 | 3.6-45 |
| Axial flow LVAD† | — | 600 |
| Centrifugal flow LVAD† | — | 150-230 |
| Blood vessel . | Shear rate (/s) . | Shear stress (Pa) . |
|---|---|---|
| Large arteries* | 300-800 | 1.4-3.6 |
| Arterioles* | 450-1600 | 2-7.2 |
| Veins* | 15-200 | 0.07-0.9 |
| Stenotic vessels* | 800-10 000 | 3.6-45 |
| Axial flow LVAD† | — | 600 |
| Centrifugal flow LVAD† | — | 150-230 |
Values for different vessels are adapted from Kroll et al74 ; mean wall shear rate/stress values are presented. Peak values could be higher.
A growing body of evidence suggests that a local shear stress in excess of 10 Pa can result in the application of forces >∼10-15 pN (piconewton = 10−12 N), which induces VWF to undergo conformational transition from a “resting” to an “active” state80 and to self-associate.71,81 Similar magnitudes of fluidic stress also promote the proteolysis by ADAMTS-13 of the cryptic Tyr1605-Met1606 peptide bond in the VWF A2-domain.72,73 In this regard, whereas VWF coexists with ADAMTS-13 in plasma, it remains mostly uncleaved unless the Tyr1605-Met1606 scissile bond is exposed by mechanical forces in solution82 or upon binding to a platelet,83 or collagen,84 or being anchored to endothelial cells.68 The force required for inducing the cleavage lies in the range of 7 to 14 pN that unfolds the VWF A2 domain.72 A slightly larger force is needed to unfold and cleave a recombinant VWF A1-A2-A3 tri-domain.73 More importantly, this magnitude of force also induces and strengthens VWF binding to platelet GP Ibα.85,86 These opposing effects of high shear stress on VWF raise the important question of which of the 2 processes (excessive cleavage by ADAMTS-13 or shear-induced binding to platelets) occurs predominantly in an LVAD-driven circulation and how the 2 processes are differentially regulated by confounding factors.
Two scenarios should be considered in elucidating the effects of mechanical forces on adhesive reactivity and cleavage when VWF is (1) subjected to shear stress in solution, because this can occur during its passage through an LVAD; and (2) attached to the biomaterials that compose the prosthetic device or to the surface of platelets or endothelial cells.
In the first scenario, VWF in flowing blood experiences periodic variations in shear forces as it tumbles through the circulation. Shear forces are at their maximal level when the protein is oriented either parallel or perpendicular to the direction of flow, and stretching forces that pull the molecule apart are at their maximum level when the protein is oriented at 45° to the flow direction.87 These oscillatory force cycles repeat every ∼10 msec when the applied shear stress is 10 Pa, with increasing shear stress proportionally decreasing the duration of force oscillation.87 Biophysical calculations based on protein geometry show that the maximal force applied to VWF during these periodic cycles is tensile in nature (rather than shear). This tensile force tends to stretch or unfold VWF multimers87 and is maximal at the geometric center of VWF when it is oriented at 45° to the direction of flow. For a VWF protomer composed of two C-terminal dimerized VWF monomers, this peak tensile force is estimated to be Fp ∼0.01 · τ, where Fp is the applied force in pN and τ is the applied shear stress in Pa.87,88 Extending this estimate to multimeric VWF with Np dimeric repeats stretched into a maximally extended linear structure, the force applied to multimeric VWF is approximately Fmulti= Fp · Np2/2.72 This derivation assumes that the force applied at the center of a fully extended VWF multimer can be estimated by summing up the hydrodynamic forces on VWF doublets that were located more outward, toward the ends of the VWF multimer. Thus, at a shear stress of 10 Pa, the peak force on a 20-mer VWF multimer would be 20 pN (0.01 × 10 × (20)2/2) applied at the midpoint of the molecule.
Studies using single-molecule fluorescence microscopy,80 small-angle neutron scattering (SANS),89 and fluorescence spectroscopy90 have suggested that VWF can undergo structural transitions under shear stresses of ∼8-10 Pa. More specifically, the fluorescence microscopy reveals a large-scale extension of VWF, with end-to-end lengths of up to 10 μm at a shear stress of >5 Pa.80 At shear stresses of >8 Pa, VWF multimers also bind to a fluorescent probe bis-ANS that recognizes nonpolar cavities exposed on the sheared protein.90 These hydrophobic pockets gradually become buried over a period of time ranging from minutes to hours, suggesting that once unfolded, proteins may not recover their native conformation immediately upon the removal of shear stress. This delayed recovery is also demonstrated in the long relaxation time of VWF fibrils returning back to their original conformation after a mechanical force applied to induce domain-unfolding was removed.52,81 A shear stress of ∼8 Pa is also necessary for VWF self-association in solution as measured using light-scattering.71 Similar magnitudes of forces enable the formation of VWF bundles or fibers in synthetic microvessels that are lined with endothelial cells.91 Thus, ∼8-10 Pa represents a critical shear threshold at which a number of investigators have noted protein structural changes measured using different techniques.
Stretching forces are also applied when VWF multimers form a bridge between 2 platelets that are tumbling together in a shear field.83,87 In this situation, the force applied to a VWF at a given shear rate would be 2 to 3 orders of magnitude greater than the force applied to free VWF in solution. Given the size of a typical human platelet, this force is estimated to be Fplat-plat(pN) ∼45 · τ.87,88 Thus, the bridging of 2 platelets by VWF at 10 Pa would result in the application of a ∼450 pN force on both the VWF multimers and the platelet GP Ibα. This force estimate may explain why VWF cleavage by ADAMTS-13 is enhanced when the VWF multimers are platelet-bound.83 The formation of VWF-crosslinked platelets through supraphysiologic shear in an LVAD would, therefore, increase force application, leading to VWF-mediated mechanotransduction/platelet activation and VWF proteolysis.71,92,93
In the second scenario, shear-driven VWF immobilization on such substrates as collagen and the biomaterial surfaces of LVADs could result in changes to VWF conformation. This was originally demonstrated using atomic force microscopy on hydrophobic substrates in which the application of wall shear stress >3.5 Pa triggered VWF unfolding.94 Extended VWF fiber meshes have also been reported on collagen substrates at shear stresses >10 Pa.84,95 These extended VWF bundles may themselves be susceptible to cleavage by ADAMTS-13, because the attachment of platelets to the immobilized VWF further enhances the force applied to both VWF and GP Ibα. The force applied to platelet-bound VWF strings can be estimated at ∼76 τ pN.88 At 10 Pa, a 760-pN force would, therefore, be applied to both VWF and the attached platelet GP Ibα, triggering both platelet mechanotransduction that activates platelets and ADAMTS-13–mediated proteolysis. Overall, when the applied shear stress exceeds the physiologic range, as is found in an LVAD-driven circulation, biophysical processes are simultaneously triggered that together promote both VWF binding to GP Ibα (and subsequent platelet activation) and its proteolysis by ADAMTS-13.
Finally, Da et al report that free hemoglobin augments platelet adhesion and microthrombus formation on an extracellular matrix at high shear stresses in a GP Ib and VWF-dependent manner.96 Hemoglobin is also reported to sterically hinder ADAMTS-13–VWF interaction.97 These findings are relevant because hemolysis often occurs in patients on LVAD support and is associated with thrombotic events.98-101 They also raise an important question regarding the potential redox-regulation of VWF reactivity and cleavage by hemoglobin, which is often called a “biological Fenton’s reagent” for its strong oxidative activity (from the heme iron).102 Oxidative stress has indeed been shown to make VWF hyper-reactive, render them resistant to ADAMTS-13, and inhibit ADAMTS-13 activity.103-106 Some of redox-sensitive residues in VWF and ADAMTS-13 are exposed by the high shear stress104 found in the LVAD-driven circulation.
LVAD-associated AVWS
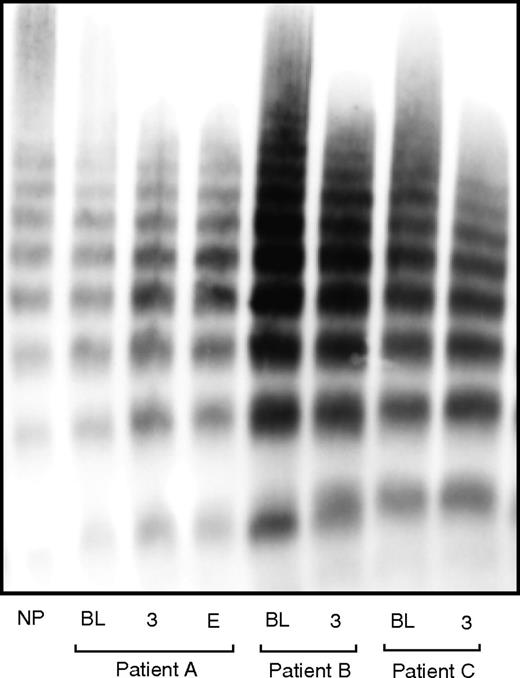
LVAD-associated AVWS has so far been defined by laboratory findings that plasma VWF from patients on LVAD support lacks large multimers (Figure 4)31,42,79,107-110 and has reduced activity in agglutinating lyophilized platelets (ristocetin cofactor activity, VWF:Rco) and in binding the collagen matrix (VWF:CB).31,79,107,108,111-115 These VWF defects are observed soon after LVAD implantation,42,43 rapidly resolve after LVAD explantation,109,111-113 and were not observed in heart transplant recipients,31,43 indicating their close association with the level and pattern of the LVAD-driven blood flow. The rapid loss of large VWF multimers and their quick recovery was also observed in a rabbit model of reversible arterial stenosis to alter blood flow.112
VWF multimer found in patients on LVAD. Examples of VWF multimer patterns found in normal plasma pooled from 32 healthy subjects and in patients on LVAD support who developed myocardial infarction (Patient A), no complication (Patient B), and severe GI bleeding (Patient C). Samples were collected before LVAD implant (BL), 3 months after implant (3), and at the time of readmission for the clinical complications (E).
VWF multimer found in patients on LVAD. Examples of VWF multimer patterns found in normal plasma pooled from 32 healthy subjects and in patients on LVAD support who developed myocardial infarction (Patient A), no complication (Patient B), and severe GI bleeding (Patient C). Samples were collected before LVAD implant (BL), 3 months after implant (3), and at the time of readmission for the clinical complications (E).
One widely held view is that the loss of VWF large multimers and reduction in VWF adhesive activity are caused by excessive cleavage of VWF by ADAMTS-13 under a persistently elevated level of shear stress in the LVAD-supported circulation. This shear-induced cleavage reduces VWF size and adhesive activity in a pattern similar to patients with type 2A VWD.31,79,107,108,116 However, this tentative mechanism has not been experimentally validated, and significant gaps remain. First, there is no direct evidence that VWF multimers are excessively cleaved by ADAMTS-13 in patients on LVAD support. Alternative VWF cleavage by other proteases such as the serine protease granzyme,117,118 plasmin,119 and Staphylococcus aureus V-8 protease,58,120 and its effect on VWF reactivity in a LVAD setting, have not been examined. For example, it has been shown that granzyme secreted from immune and cytotoxic lymphocytes in conditions of inflammation proteolytically reduces VWF adhesive activity117-119 and disrupts VWF–FVIII interaction.118 The serine protease plasmin cleaves VWF fibrils that are resistant to ADAMTS-13.119 Second, VWF antigen was significantly increased in all patients,79,108,109 a condition that is not common in patients with type 2 VWD.121,122 The question is whether the loss of large VWF multimers can be compensated for by an increase in VWF antigen. Third, the loss of large VWF multimers has been observed in nearly all patients, but only a small portion of them have clinically relevant bleeding. More importantly, neither the loss of large VWF multimers nor a reduced VWF:Rco activity is associated with bleeding, thrombosis, or the need for transfusion in patients on LVAD support.79,87,109,114,123 Although this lack of association may be attributed to clinical variations among patients, it is also possible that AVWS constitutes a baseline condition that has minimal clinical consequences on its own, and that requires a trigger or additive event to propagate bleeding and/or thrombosis in patients on LVAD support. Fourth, while it facilitates VWF cleavage by ADAMTS-13,72,73,82 a high fluid shear stress or a tensile force also unfolds VWF to bind and activate platelets,74,81,124-126 potentially leading to a consumptive deficiency in large VWF multimers, similar to patients with type 2B VWD. This shear-induced platelet aggregation depends on the attachment of large VWF multimers to GP Ibα and the activation of integrin αIIbβ3.125,127,128 This gain-of-function VWF phenotype can not only remove large VWF multimers from the circulation, but also occlude cerebral and GI microvessels, producing punctate injury, bleeding, and AVM. In this case, a patient with deficient large VWF multimers may be prone to thrombosis, not bleeding. If the theory of shear-induced platelet aggregation is to be credence, one may consider that shear- and/or oxidative stress-mediated VWF binding to platelets is effectively prevented in most patients by therapeutic agents that block platelet ADP receptors (clopidogrel) and/or integrin αIIbβ3 (tirofiban, eptifibatide).129,130 The patients who develop bleeding or thromboembolism may have an inadequate therapeutic blockade of platelets in terms of the dosage, type, or protocol used during their care. Furthermore, aspirin is widely used to inhibit platelet activation in patients on LVAD support, but has been found to be ineffective in vitro to prevent shear-induced and VWF-mediated aggregation of platelets.125 A distinction between excessive VWF cleavage (loss-of-function) and enhanced VWF binding to platelets (gain-of-function) may, therefore, provide further insights into the causes of LVAD-induced loss of large VWF multimers and its association with bleeding and thrombosis.
Conclusion
The evidence in support of the excessive cleavage of large VWF multimers by ADAMTS-13 as a primary cause of LVAD-associated bleeding remains circumstantial. A large body of clinical and laboratory evidence suggests that the LVAD-associated loss of large VWF multimers can also be caused by shear- and oxidative stress–induced VWF binding to platelets. This conjecture is consistent with a recent report that thromboembolic events were 7.4 times as likely to occur in LVAD patients who had experienced prior GI bleeding.121 Defining this phenotypic diversity may hold the key to understanding the contribution of AVWS to the bleeding and thrombotic complications associated with LVAD implantation. It may also be essential for devising effective preventative and therapeutic strategies for the debilitating complications.
Acknowledgments
This work is supported by National Institute of Health, National Heart, Lung, and Blood Institute grants HL77258 (S.N.), and HL71895 and HL125957 (J.-f.D.).
Authorship
A.N., S.N., and J.-f.D. conducted background research, and all authors wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jing-fei Dong, BloodWorks Research Institute, 1551 Eastlake Ave East, Seattle, WA 98102; e-mail: jfdong@bloodworksnw.org.