In this issue of Blood, Vasu et al show that a novel, Fc-engineered CD33 antibody, BI 836858, promotes natural killer (NK) cell–mediated antibody-dependent cellular cytotoxicity (ADCC) with in vitro activity against both acute myeloid leukemia (AML) cell lines and primary AML blasts.1
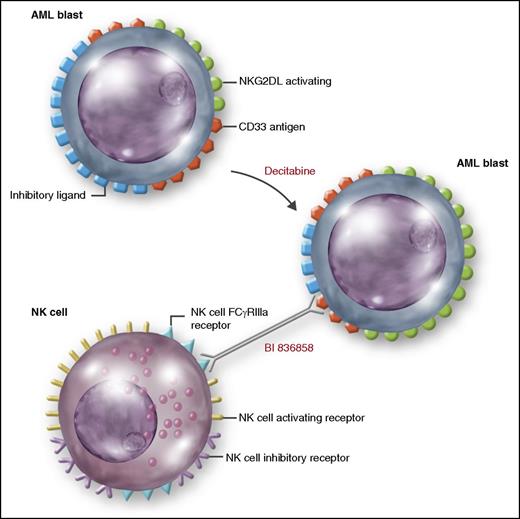
Decitabine may have immunomodulatory effects. Vasu et al show that decitabine significantly increases NKG2DL on AML blasts resulting in higher BI 836858–mediated ADCC postdecitabine when compared with predecitabine treatment. Professional illustration by Somersault18:24.
Decitabine may have immunomodulatory effects. Vasu et al show that decitabine significantly increases NKG2DL on AML blasts resulting in higher BI 836858–mediated ADCC postdecitabine when compared with predecitabine treatment. Professional illustration by Somersault18:24.
Serial evaluation in patients treated with a 10-day course of decitabine demonstrated higher BI 836858–mediated ADCC at day 28 post–decitabine therapy compared with pretreatment that was associated with significant upregulation of ligands to NK-activating receptor NKG2D (NKG2DL) in day 28 postdecitabine samples when compared with pretreatment. This study highlights 2 novel strategies relevant to antibody-based approaches in AML: (1) the ability of an unconjugated antibody engineered for increased binding of FcγRIIIa and low nanomolar affinity to human CD33 to induce significant antileukemic cytotoxicity by exploiting NK cell–mediated ADCC and (2) the ability to exploit immunomodulatory changes induced by DNA-methyltransferase inhibitors (DNMTi’s) to enhance NK cell activation and induce NK-mediated activity against AML blasts.
CD33 is expressed by myeloid blast cells in >80% of patients with AML suggesting that antibodies to CD33 may have therapeutic activity in the treatment of AML. Several CD33 antibody-drug conjugates (ADCs), such as gemtuzumab ozogamicin (GO), AVE9633, and SGN-33A, and unconjugated humanized antibodies, such as lintuzumab (SGN-33), have been evaluated in the treatment of AML.2-4 GO, a humanized anti-CD33 monoclonal antibody (mAb) covalently linked to a potent toxin, calicheamicin, was voluntarily withdrawn from the US market in 2010 after a phase 3 trial showed a trend toward increased induction mortality in the GO arm.5 Several other large randomized trials examining the potential efficacy of GO in combination with traditional cytotoxic induction therapy in AML demonstrated improved outcomes6 particularly among patients with intermediate or good-risk cytogenetics. SGN-33A, a next-generation ADC conjugated to a novel payload (pyrrolobenzodiazepine), has demonstrated impressive in vitro targeted cell killing and encouraging activity both as a single agent and in combination regimens in early clinical trials.4 These ADCs depend heavily on inducing apoptosis of the target cells by delivery of a toxic payload.
Lintuzumab, an unconjugated CD33-directed antibody, on the other hand exerted its antileukemic activity not through direct killing but via ADCC or antibody-dependent cellular phagocytosis mediated by NK cells, complement-dependent cytotoxicity, and inhibition of inflammatory cytokine production. Lintuzumab produced complete remissions (CRs) in elderly patients, but randomized studies did not show a survival benefit when it was added to other agents.7 BI 836858, a fully human nonconjugated anti-CD33 antibody, essentially relies on these same therapeutic concepts. However, BI 836858 was developed as an Fc-engineered mAb with enhanced affinity to FcγRIIIA receptor on NK cells and demonstrates decelerated internalization kinetics compared with prior CD33-directed mAb’s. These 2 characteristics may explain why BI 836858 induces a more robust NK cell–mediated ADCC than lintuzumab as demonstrated in the reported study.
DNMTi’s (decitabine and 5-azacytidine) are used commonly for the treatment of less fit, older patients with AML in the United States and Europe. They exert their antineoplastic activity by direct incorporation into DNA with subsequent inhibition of DNA methyltransferase resulting in reexpression of tumor suppressor genes. DNMTi’s have been combined with mAb’s with varying degrees of success. Two studies combined DNMTi’s with GO based on anticipated synergy between DNMTi’s and GO found in preclinical studies demonstrating that DNMTi’s induced increased CD33 expression, decreased P-glycoprotein expression, and enhanced DNA intercalation by calicheamicin. Nand et al evaluated a combination of 5-azacytidine and GO in patients with newly diagnosed myelodysplastic syndrome (MDS) and AML.8 Responses including CR plus complete remission with incomplete recovery of counts (CRi) were seen in 44% and 35% of the good- and poor-risk patients, respectively. Median overall survival was 11 months in both risk groups. In a phase 2 study, Daver et al reported that decitabine in combination with GO improved response rate among patients with untreated AML ≥60 years of age who were unfit for chemotherapy, AML evolving from treated MDS or previously treated MDS or myelofibrosis.9 However, this did not translate into improved survival when compared with historical data.
DNMTi’s have been shown to modulate antitumor NK immune responses including upregulation of NKG2DL expression on AML blasts and enhanced responsiveness to activating stimuli by inducing transcription of genes involved in NK cell reactivity. AML induces an imbalance between NK receptors, which favors the inhibitory receptors resulting in impaired NK antileukemic activity.10 Upregulation of the NK cell activating receptors or activating receptor ligands may restore the balance in favor of NK cell activation and resuscitate NK cytotoxicity. It has been demonstrated that 5-azacytidine significantly enhanced the ability of the earlier generation unconjugated anti-CD33 mAb (lintuzumab) to promote tumor cell killing through NK-mediated ADCC. Initial results from an ongoing phase 2 study combining SGN-CD33A with a DNMTi (azacytidine or decitabine) for elderly, treatment-naïve patients with AML are promising with a CR plus CRi rate of 65% and 8-week mortality of 4%, and with 85% of treated patients having a ≥50% reduction in blasts.4 Vasu et al note a similar in vitro synergy with significantly higher NKG2DL expression and higher BI 836858–mediated ADCC at day 28 when compared with predecitabine treatment (see figure). When the NKG2DL receptor was blocked, the enhanced ADCC activity was abrogated suggesting upregulation of NKG2DL as the mode of synergism. A clinical trial evaluating this combination will begin later this year and will likely provide further insight to the benefits of such a strategy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.