Key Points
Sialic acids are critical for factor H–mediated complement regulation on endothelial cells, erythrocytes, and platelets.
Impaired ability of factor H mutants to simultaneously bind sialic acid and C3b on cells explains their association with aHUS.
Abstract
Uncontrolled activation of the complement system against endothelial and blood cells is central to the pathogenesis of atypical hemolytic uremic syndrome (aHUS). aHUS patients frequently carry mutations in the inhibitory complement regulator factor H (FH). Mutations cluster in domains 19 and 20 (FH19-20), which are critical for recognizing self surfaces. On endothelial cells, binding of FH is generally attributed to heparan sulfate. This theory, however, is questioned by the puzzling observation that some aHUS-associated mutations markedly enhance FH binding to heparin and endothelial cells. In this article, we show that, instead of disturbed heparin interactions, the impaired ability of C-terminal mutant FH molecules to recognize sialic acid in the context of surface-bound C3b explains their pathogenicity. By using recombinant FH19-20 as a competitor for FH and measuring erythrocyte lysis and deposition of complement C3b and C5b-9 on endothelial cells and platelets, we now show that several aHUS-associated mutations, which have been predicted to impair FH19-20 binding to sialic acid, prevent FH19-20 from antagonizing FH function on cells. When sialic acid was removed, the wild-type FH19-20 also lost its ability to interfere with FH function on cells. These results indicate that sialic acid is critical for FH-mediated complement regulation on erythrocytes, endothelial cells, and platelets. The inability of C-terminal mutant FH molecules to simultaneously bind sialic acid and C3b on cells provides a unifying explanation for their association with aHUS. Proper formation of FH-sialic acid-C3b complexes on surfaces exposed to plasma is essential for preventing cell damage and thrombogenesis characteristic of aHUS.
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a rare thrombotic microangiopathy characterized by thrombocytopenia, hemolysis, and kidney failure.1 Central to the disease is dysregulation of the complement system,2 a network of serum proteins involved in, for example, elimination of invading pathogens and damaged self cells (reviewed by Ricklin et al3 ). The most frequent genetic abnormality in aHUS is a mutation in the complement regulator factor H (FH).4 Mutations cluster in the C-terminal domains 19 and 20 of FH (FH19-20),5-7 which are critical for directing complement regulation onto self surfaces.8,9 The identity of the binding partner of FH on different cells is as yet unconfirmed.
All cells in contact with plasma are continuously exposed to a low level of complement activation, and the regulator FH participates in preventing amplification of this activity. On endothelial cells, the structure responsible for attracting FH onto the surface is generally considered to be the glycosaminoglycan heparan sulfate.10 FH binds to the highly sulfated form of this polyanion, heparin, with relatively high affinity. The presence of heparin on particles has been shown to enhance FH-assisted inactivation of C3b bound to the particles.11 Treatment of human kidney glomerulus sections with heparinases reduces both FH and, importantly, FH19-20 binding.10 However, the importance of heparan sulfate for FH binding to endothelium is challenged by the observation that some aHUS-related FH mutants show increased binding to heparin12-14 and to endothelial cells.13 Enhanced FH binding to cells would intuitively be expected to result in more efficient complement regulation on cells. Accordingly, it is unclear how these mutations are associated with aHUS.
One characteristic feature of aHUS is intravascular hemolysis. In this syndrome, erythrocyte rupture is generally considered to be mechanical,4 but a role of complement cannot be ruled out. On erythrocytes, the main candidate binding partner of FH is sialic acid.15,16 The affinity of FH for sialic acid alone is very weak,17 and binding has been reported only in a buffer of 0.10 physiological ionic strength.18 However, sialic acid has been observed only to enhance FH binding to surface-deposited C3b. For example, removal of 70% of sialic acid from C3b-coated sheep erythrocytes (Es) leads to a 60% to 80% decrease in FH binding.16 Similarly, the more sialic acid is removed from Es, the more FH is needed to dissociate Bb from C3bBb.15 In C3b-coated liposomes, the sialic acid–containing glycolipid GM1 increased binding of FH.19 Experiments with gonococci showed that the FH site responsible for interactions with sialic acid lies in the C terminus of the protein.20 Recently, the crystal structure of FH19-20 bound to sialic acid and the C3d part of C3b was determined, and the residues of FH responsible for interactions with sialic acid were uncovered (Figure 1A).17,21,22
FH19-20 residues involved in binding to C3b and sialic acid. Shown are mesh-and-sphere representations of the FH19-20 structure (Protein Data Bank [PDB] ID code 2G7I)14 from two different angles. (A) Binding sites for C3b/C3d and sialic acid. The main interface residues forming the C3b binding site (N1117, Q1139, Y1142, P1166, K1188, and Y1190)21,22 are shown in green. The residues likely to have an impact on sialic acid binding (I1169, R1182, T1184, L1189, S1191, G1194, V1197, E1198, F1199, and R1215)17 are shown in dark yellow. Residues of specific relevance to this study (T1184, L1189 and E1198) have been highlighted with orange. (B) Location of the residues that were mutated in the control constructs. Whereas the aHUS-associated mutations T1184R, L1189R, and E1198A lead to increased FH19-20 affinity for heparin, other mutations have been reported to either decrease (R1182A, K1186A, and R1215Q) or have no effect (W1157L, W1183L) on the interaction.12,13 Residues R1182, W1183, K1186, and R1215 are depicted in blue. Residue W1157 is invisible because it is buried within the domain 19.
FH19-20 residues involved in binding to C3b and sialic acid. Shown are mesh-and-sphere representations of the FH19-20 structure (Protein Data Bank [PDB] ID code 2G7I)14 from two different angles. (A) Binding sites for C3b/C3d and sialic acid. The main interface residues forming the C3b binding site (N1117, Q1139, Y1142, P1166, K1188, and Y1190)21,22 are shown in green. The residues likely to have an impact on sialic acid binding (I1169, R1182, T1184, L1189, S1191, G1194, V1197, E1198, F1199, and R1215)17 are shown in dark yellow. Residues of specific relevance to this study (T1184, L1189 and E1198) have been highlighted with orange. (B) Location of the residues that were mutated in the control constructs. Whereas the aHUS-associated mutations T1184R, L1189R, and E1198A lead to increased FH19-20 affinity for heparin, other mutations have been reported to either decrease (R1182A, K1186A, and R1215Q) or have no effect (W1157L, W1183L) on the interaction.12,13 Residues R1182, W1183, K1186, and R1215 are depicted in blue. Residue W1157 is invisible because it is buried within the domain 19.
Although erythrocytes and platelets are also affected, endothelial injury is generally accepted as the central factor among the events leading to thrombotic microangiopathy.23 Endothelial cells are protected from complement by several membrane regulators, but occurrence of aHUS featuring mutations in the regulator FH shows the importance of this serum-based complement inhibitor in protecting the endothelium. As with erythrocytes, the glycocalyx of endothelial cells is rich in sialic acid.24 This raises the prospect of sialic acid involvement in complement regulation on endothelial cells.17 The recently published structure of FH bound to sialic acid suggests that the mutations T1184R, L1189R, and E1198A (known to enhance FH binding to heparin)12,13 could instead impair FH interactions with sialic acid.17 In this work, we showed that the mutations L1189R and E1198A impaired the ability of FH to recognize sialic acid on C3b-bearing surfaces. We also showed that sialic acid is critical for FH-mediated complement regulation not only on erythrocytes but also on endothelial cells and platelets. We propose that failure of FH molecules with C-terminal mutations to form a complex consisting of sialic acid and C3b on self cells explains their association with aHUS.
Methods
The study was approved by the Institutional Review Board of the University of Helsinki. Written informed consent was obtained from all donors in accordance to the Declaration of Helsinki.
Hemolysis
Es (Bio Karjalohja) or human erythrocytes (Eh) were washed with an N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (pH 7.4), 144 mM NaCl, 2.5 mM MgCl2, and 10 mM EGTA. In assays with Es, 3 × 106 cells were pre-incubated with increasing concentrations of FH19-20 fragments13 (0.6 to 18 µM), followed by the addition of normal human serum (NHS) to a final concentration of 15%; total volume was 60 µL. With Eh, 2 × 106 cells, 20 µM FH19-20 fragments, and 33% NHS were used. Erythrocytes were incubated at 37°C for 20 minutes, followed by centrifugation and immediate separation of supernatants. Absorbances were measured at 414 nm. Hemolysis in each sample was compared with that of water-induced total lysis.
MST
FH (14 nM; Complement Technologies) or Maackia amurensis lectin II (MAL II; 5 nM; Vector Laboratories), both labeled with NT-647 (NanoTemper Technologies), was incubated with 3′sialyllactose (1.5 µM to 50 mM) in 50% phosphate-buffered saline (PBS) containing 0.1% gelatin. Samples were loaded into glass capillaries, and thermophoresis was performed by using Monolith NT.115Pico with light emitting diode and microscale thermophoresis (MST) powers set to 10% and 80%, respectively.
Inhibition of FH binding to C3b-bearing Es and beads
C3b was deposited on Es and magnetic beads (Pierce) by using purified C325 and factors B and D (Complement Technology) as previously described.25 C3b deposition on the resulting EsC3b and C3b beads was verified by showing binding of NT-647–labeled anti-C3c antibody (Dako) by flow cytometry (CyAn ADP Analyzer).
Sialic acid was removed from EsC3b (1 × 108 cells in 200 µL) by incubation with 1000 U sialidase (α2-3,6,8 neuraminidase; New England Biolabs) at 37°C for 30 minutes in PBS. Cells were washed with gelatin-PBS. Removal of sialic acid was assessed by measuring binding of NT-647–labeled MAL II by flow cytometry. The level of remaining C3b deposition on the cells after sialidase treatment was probed as described above.
In the actual binding assays with EsC3b, 3 × 105 sialidase-treated or untreated EsC3b in 0.1% gelatin-PBS were mixed with FH19-20 (30 µM) or PBS. NT-647–labeled FH (0.1 µM) was added, followed by a 60-second incubation and flow cytometry analysis. In the dose-response experiment, FH19-20 concentrations were 0, 3, 6, 12, 23, or 35 µM. C3b bead assays were performed similarly, except that 8 × 106 beads, 0.03 µM NT-647–labeled FH, and 0.8 µM FH19-20 were used.
Endothelial cell assays
Human umbilical vein endothelial cells (HUVECs,26 a kind gift from Mikko Helenius, University of Helsinki, Helsinki, Finland) or human glomerular endothelial cells (Icelltis) were passaged 4 or 5 times, detached, and washed with gelatin-PBS. Cells were activated by incubating them with 10 ng/mL tumor necrosis factor α (TNF-α) (PeproTech) for at least 12 hours.27
For the binding assays, C3b-bearing cells were prepared by activating complement on cells (1 × 106) in 25% NHS with the anti-CD59 antibody YHT53.1.28 C3b deposition was confirmed as described above. In the MAL II-FH binding competition assay, C3b-bearing HUVECs (1 × 104) were incubated with 0 to 2 µM MAL II and 0.1 µM NT-647–labeled FH for at least 15 minutes, followed by flow cytometry analysis. The assays that analyzed competition of FH binding by FH fragments were performed with 10 µM FH19-20 or 100 µM FH6-8.29
To set up a calcein-release assay, HUVECs (3 × 106 in gelatin-PBS) were incubated with 25 µM calcein AM (BD Pharmingen) at 37°C for 30 minutes, followed by washing. Cells (1 × 104) were incubated with anti-CD59 antibody (0 to 0.13 µM) in 15% NHS for 15 minutes. Total lysis in the control sample was performed with Triton X-100. Supernatant samples were mixed 1:1 with CytoTox-ONE (Promega) reagent to determine possible lactate dehydrogenase (LDH) liberation. Calcein fluorescence was measured at excitation/emission wavelengths of 485/520 nm and that of an indicator for LDH at 540/590 nm (FLUOstar OPTIMA; BMG Labtech).
In the actual functional studies, 4 × 104 cells were mixed with anti-CD59 antibody (0.01 µM), FH19-20 (6 µM), and NHS (15%) at 4°C. Cells were transferred to 37°C for 14 minutes. Only calcein fluorescence was measured.
Sialidase-treated HUVECs were prepared by incubating 1.5 × 106 cells in 200 µL of PBS containing sialidase (1500 U) at 37°C for 30 minutes and washed. Assessment of sialic acid removal was determined as described above.
In the C3b deposition assay, HUVECs (4 × 104) were incubated with FH19-20 fragments (20 µM) or PBS. C3 (3 µM) labeled with DyLight 488 (Thermo Scientific) was added, followed by 20 µL of NHS (final concentration, 33%). Samples were incubated at 37°C for 30 minutes. Gelatin-PBS was added, and samples were analyzed by flow cytometry.
Platelets
Washed human platelets were prepared as previously described.30 To remove sialic acid, 2 × 108 platelets in 200 µL citrate buffer30 was incubated with sialidase (1500 U) for 30 minutes at 37°C. Platelets were washed first with citrate buffer and then modified Tyrode’s buffer.30 Assessment of sialic acid removal was determined as described above.
For complement activation assays, C3b deposition experiments similar to those for HUVECs were performed, except that platelets (2 × 106) were incubated with FH19-20 fragments, labeled C3, and NHS for only 15 minutes. To analyze platelet activation, P-selectin expression was determined by measuring binding of phycoerythrin/Cy5-labeled anti-P-selectin antibody (BioLegend) by flow cytometry.
Results
Mutations impair the ability of FH19-20 to prevent FH function on erythrocytes
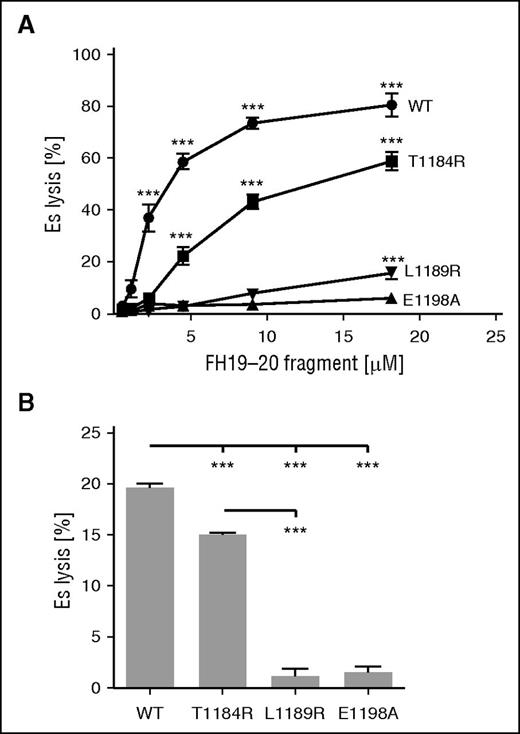
In aHUS, C-terminal mutations of FH prevent proper complement regulation on cells.8,9 We chose to study the effects of mutations on FH function by comparing the abilities of mutant and wild-type (wt) FH19-20 to prevent FH from protecting erythrocytes from complement-mediated lysis in serum. We observed that the mutation E1198A abolished the ability of FH19-20 to antagonize FH function on both Es and Eh (Figure 2). The same was true of L1189R (Figure 2), as reported previously.12 The failure of the mutants L1189R and E1198A to inhibit FH function was not the result of protein misfolding, because all FH19-20 fragments efficiently competed with FH in binding to C3b beads (Figure 3). In contrast to earlier work showing no effect for the mutation T1184R, we observed that it inhibited FH19-20 function slightly on both Es and Eh (Figure 2). These results indicate that mutations L1189R and E1198A severely impair FH function on sialic acid-bearing erythrocytes.
Effect of mutations on the ability of FH19-20 in preventing FH-mediated protection of erythrocytes from complement-mediated lysis in serum. (A) Es were incubated in EGTA-containing NHS (15%) in the presence of increasing concentrations of wt or mutant FH19-20. (B) Eh were incubated with wt or mutant FH19-20 (20 µM) in 33% NHS with EGTA. (A-B) Hemolysis in each sample was determined by measuring the absorbance of the supernatant at 414 nm compared with that obtained with total lysis performed with water. The assays were performed (A) 6 or (B) 3 times. Shown are average values ± standard deviation (SD). One-way analysis of variance (ANOVA) with Tukey’s multiple comparison posttests was performed. ***P < .001.
Effect of mutations on the ability of FH19-20 in preventing FH-mediated protection of erythrocytes from complement-mediated lysis in serum. (A) Es were incubated in EGTA-containing NHS (15%) in the presence of increasing concentrations of wt or mutant FH19-20. (B) Eh were incubated with wt or mutant FH19-20 (20 µM) in 33% NHS with EGTA. (A-B) Hemolysis in each sample was determined by measuring the absorbance of the supernatant at 414 nm compared with that obtained with total lysis performed with water. The assays were performed (A) 6 or (B) 3 times. Shown are average values ± standard deviation (SD). One-way analysis of variance (ANOVA) with Tukey’s multiple comparison posttests was performed. ***P < .001.
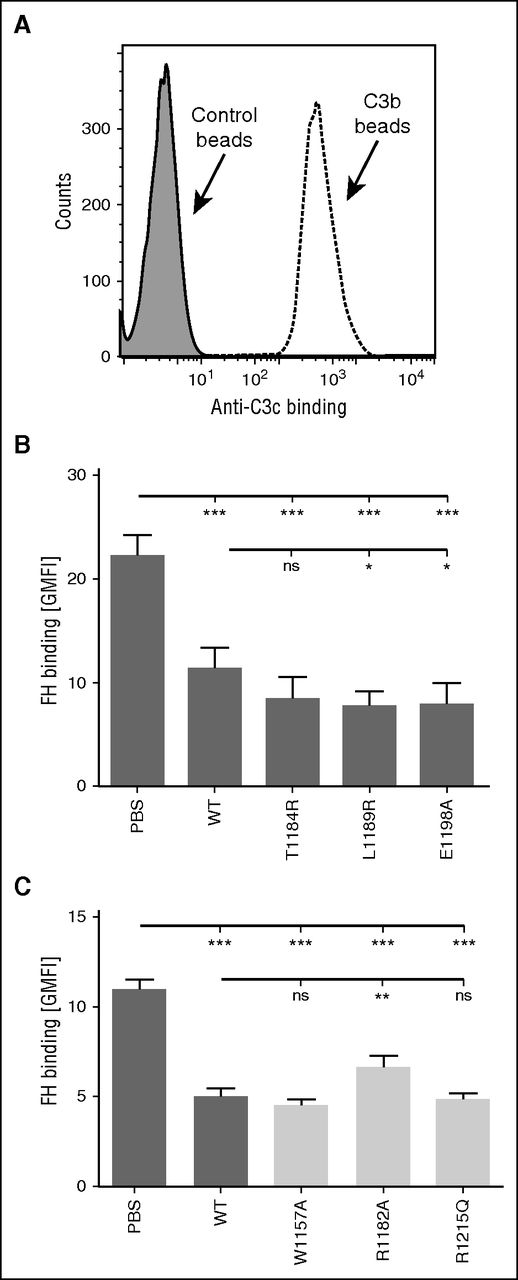
Effect of C-terminal mutations in FH binding to C3b deposited on beads. (A) C3b was deposited on beads by using purified C3 and factors B and D. C3b deposition was verified by showing increased binding of anti-C3c antibody by flow cytometry. (B-C) FH binding to C3b-bearing beads was competed with wt and several mutant FH19-20 fragments. FH was fluorescently labeled, and binding was analyzed by flow cytometry. (B) Data on FH binding in the presence of FH19-20 mutants of specific interest to this study are shown by dark gray–shaded bars; (C) data for control mutants are shown by light gray–shaded bars. The assays were performed at least 4 times. Shown are average geometric mean fluorescence intensity (GMFI) values (± SD). One-way ANOVA with Tukey’s multiple comparison posttests was performed. *P < .05; **P < .01; ***P < .001. ns, not significant.
Effect of C-terminal mutations in FH binding to C3b deposited on beads. (A) C3b was deposited on beads by using purified C3 and factors B and D. C3b deposition was verified by showing increased binding of anti-C3c antibody by flow cytometry. (B-C) FH binding to C3b-bearing beads was competed with wt and several mutant FH19-20 fragments. FH was fluorescently labeled, and binding was analyzed by flow cytometry. (B) Data on FH binding in the presence of FH19-20 mutants of specific interest to this study are shown by dark gray–shaded bars; (C) data for control mutants are shown by light gray–shaded bars. The assays were performed at least 4 times. Shown are average geometric mean fluorescence intensity (GMFI) values (± SD). One-way ANOVA with Tukey’s multiple comparison posttests was performed. *P < .05; **P < .01; ***P < .001. ns, not significant.
Sialic acid is important for FH binding to erythrocytes
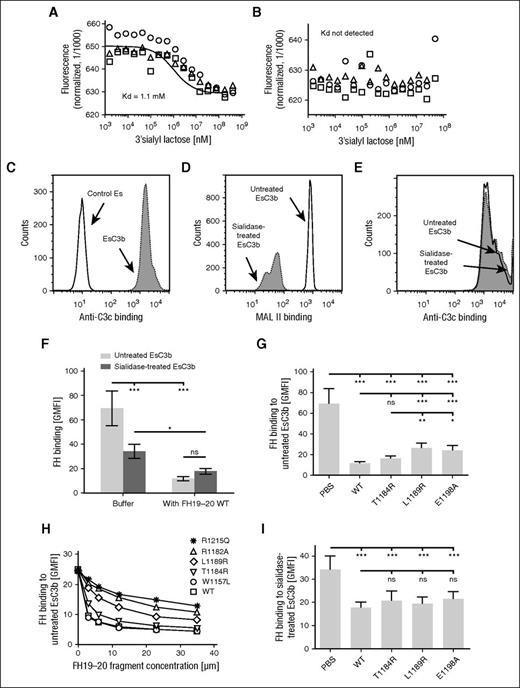
To study the very weak affinity of FH for sialic acid,17 we chose to use MST technique. Whereas a dissociation constant of 1.1 mM for the lectin MAL II in binding to the sialic acid–containing 3′sialyllactose could be determined (Figure 4A), no interaction between FH and 3′sialyllactose was observed (Figure 4B). This result explains the lack of reports on FH binding to sialic acid in physiological buffers.
Sialic acid enhances FH binding to EsC3b. (A-B) Binding of FH to sialic acid in the absence of C3b was studied by using the MST technique. Fluorescently labeled (A) MAL II or (B) FH was mixed with increasing concentrations of the sialic acid containing compound 3′sialyllactose followed by thermophoresis. Results of experiments performed in triplicate are represented as spheres, triangles, and squares. Binding data for MAL II was fitted with NanoTemper software to obtain the binding isotherm and the respective dissociation constant (Kd). (C) To study FH binding to sialic acid in the context of C3b, C3b was deposited on Es by using purified C3 and factors B and D. C3b deposition on Es was verified by showing increased binding of anti-C3c antibody using flow cytometry. (D) Erythrocytes with C3b deposition on them (EsC3b) were treated with sialidase to remove sialic acid from their surfaces. Efficiency of the sialidase treatment was verified by showing decreased binding of MAL II lectin to sialidase-treated cells by flow cytometry. (E) The level of C3b deposition on EsC3b was not affected by treatment with sialidase, as judged by similar binding of anti-C3c antibody to untreated and sialidase-treated EsC3b cells. (C-E) Representative histograms of assays performed 3 to 6 times. (F-I) Binding of FH to untreated and sialidase-treated EsC3b was competed with wt and mutant FH19-20 fragments. Binding of fluorescently labeled FH was analyzed by flow cytometry. (F) Removal of sialic acid from EsC3b decreased FH binding to the cells. The wt FH19-20 had a major inhibitory effect on FH binding to untreated EsC3b but only a small effect on FH binding to the sialidase-treated EsC3b. (G) FH binding to untreated EsC3b in the presence of wt and mutant FH19-20. (H) A dose-response study of the ability of various FH19-20 mutants to antagonize FH binding to untreated EsC3b. Some controls (FH19-20 mutants W1157L, R1182A, and R1215Q) were included in the assay. (I) FH binding to sialidase-treated EsC3b in the presence of wt and mutant FH19-20. The assays were performed (H) 3 or (F-G, I) 5 times. Shown are average GMFI values (± SD). One-way ANOVA with Tukey’s multiple comparison posttests was performed.*P < .05; **P < .01; ***P < .001.
Sialic acid enhances FH binding to EsC3b. (A-B) Binding of FH to sialic acid in the absence of C3b was studied by using the MST technique. Fluorescently labeled (A) MAL II or (B) FH was mixed with increasing concentrations of the sialic acid containing compound 3′sialyllactose followed by thermophoresis. Results of experiments performed in triplicate are represented as spheres, triangles, and squares. Binding data for MAL II was fitted with NanoTemper software to obtain the binding isotherm and the respective dissociation constant (Kd). (C) To study FH binding to sialic acid in the context of C3b, C3b was deposited on Es by using purified C3 and factors B and D. C3b deposition on Es was verified by showing increased binding of anti-C3c antibody using flow cytometry. (D) Erythrocytes with C3b deposition on them (EsC3b) were treated with sialidase to remove sialic acid from their surfaces. Efficiency of the sialidase treatment was verified by showing decreased binding of MAL II lectin to sialidase-treated cells by flow cytometry. (E) The level of C3b deposition on EsC3b was not affected by treatment with sialidase, as judged by similar binding of anti-C3c antibody to untreated and sialidase-treated EsC3b cells. (C-E) Representative histograms of assays performed 3 to 6 times. (F-I) Binding of FH to untreated and sialidase-treated EsC3b was competed with wt and mutant FH19-20 fragments. Binding of fluorescently labeled FH was analyzed by flow cytometry. (F) Removal of sialic acid from EsC3b decreased FH binding to the cells. The wt FH19-20 had a major inhibitory effect on FH binding to untreated EsC3b but only a small effect on FH binding to the sialidase-treated EsC3b. (G) FH binding to untreated EsC3b in the presence of wt and mutant FH19-20. (H) A dose-response study of the ability of various FH19-20 mutants to antagonize FH binding to untreated EsC3b. Some controls (FH19-20 mutants W1157L, R1182A, and R1215Q) were included in the assay. (I) FH binding to sialidase-treated EsC3b in the presence of wt and mutant FH19-20. The assays were performed (H) 3 or (F-G, I) 5 times. Shown are average GMFI values (± SD). One-way ANOVA with Tukey’s multiple comparison posttests was performed.*P < .05; **P < .01; ***P < .001.
Although too weak on its own, sialic acid nevertheless increases FH affinity for surface-bound C3b.15,16,19 Thus, we next studied FH-sialic acid interactions with C3b-bearing Es (Figure 4C). We observed that the mutations L1189R and E1198A (but not T1184R) impaired the ability of FH19-20 to prevent FH binding to EsC3b (Figure 4G). The effect was even clearer in a dose-response analysis (Figure 4H). Several mutants with normal or impaired affinity for heparin (Figure 1B) were included in the experiment as controls, and a detrimental effect for mutation R1215Q was observed (Figure 4H). These results are in line with earlier data on the function of these mutants with C3b-bearing erythrocytes.12
Removal of sialic acid from EsC3b caused a major decrease in binding of FH to EsC3b (Figure 4D,F), as expected on the basis of earlier reports.15,16 This was not the result of differences in the level of C3b on cells, because EsC3b with and without sialic acid showed equal deposition of C3b (Figure 4E). Furthermore, sialidase treatment affected the relative binding of the wt FH19-20 fragment compared with the mutants. Whereas on normal EsC3b, the wt FH19-20 was significantly more efficient than the mutants L1189R and E1198A in competing with FH in binding to cells, in the absence of sialic acid the superiority of wt FH19-20 over the mutants was completely lost (Figure 4I). Taken together, these results indicate that FH binds simultaneously to sialic acid and C3b on erythrocytes, and that several aHUS-related mutations impair formation of this ternary complex.
Sialic acid is important for complement regulation on endothelial cells
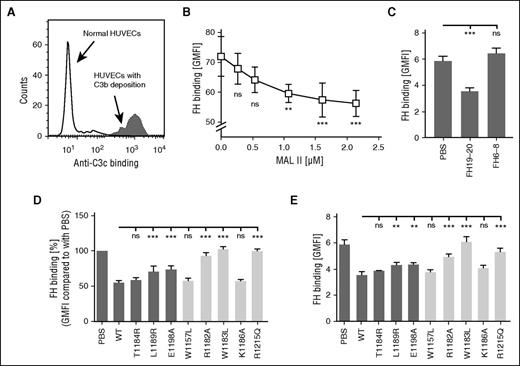
Thrombotic microangiopathies feature endothelial cell injury and, regarding aHUS, activation and regulation of the complement cascade on endothelium is of special interest. To determine the as yet unknown role of sialic acid in controlling complement on endothelial cells, we started by studying FH binding to C3b-bearing cells (Figure 5A). First, it was observed that the sialic acid–binding lectin MAL II dose-dependently inhibited the interaction between FH and C3b-bearing HUVECs (Figure 5B). We observed that, similar to the results from the erythrocyte experiments, the L1189R and E1198A (but not T1184R) mutations impaired the ability of FH19-20 to prevent FH binding to both HUVECs (Figure 5D) and glomerular endothelial cells (Figure 5E) with C3b deposits. Results with the control mutants also paralleled EsC3b data (Figure 5D-E). Unlike FH19-20, FH6-8 had no effect on FH binding to C3b-bearing glomerular cells (Figure 5C).
FH interacts with sialic acid on endothelial cells. (A) C3b was deposited on HUVECs by incubating cells in 25% NHS and using an anti-CD59 antibody to initiate complement activation. Deposition of C3b on cells was verified by showing increased binding of anti-C3c antibody by flow cytometry. (B-E) Binding of FH to C3b-bearing endothelial cells was competed with various proteins. Binding of fluorescently labeled FH was analyzed by flow cytometry. (B) A dose-response study of FH binding to TNF-α–activated C3b-bearing HUVECs in the presence of increasing concentrations of the lectin MAL II. (C) With 10 µM FH19-20, FH binding to TNF-α–activated C3b-bearing glomerular endothelial cells was inhibited, but it was not inhibited by 100 µM FH6-8. FH binding to (D) C3b-bearing HUVECs or to (E) TNF-α–activated C3b-bearing glomerular endothelial cells in the presence of wt and mutant FH19-20 fragments. Data on mutants of specific interest to this study are shown by dark gray–shaded bars, and data on controls are shown by light gray–shaded bars. Shown are average values (± SD) of experiments performed at least 3 times. One-way ANOVA with Tukey’s multiple comparison posttests was performed. **P < .01; ***P < .001.
FH interacts with sialic acid on endothelial cells. (A) C3b was deposited on HUVECs by incubating cells in 25% NHS and using an anti-CD59 antibody to initiate complement activation. Deposition of C3b on cells was verified by showing increased binding of anti-C3c antibody by flow cytometry. (B-E) Binding of FH to C3b-bearing endothelial cells was competed with various proteins. Binding of fluorescently labeled FH was analyzed by flow cytometry. (B) A dose-response study of FH binding to TNF-α–activated C3b-bearing HUVECs in the presence of increasing concentrations of the lectin MAL II. (C) With 10 µM FH19-20, FH binding to TNF-α–activated C3b-bearing glomerular endothelial cells was inhibited, but it was not inhibited by 100 µM FH6-8. FH binding to (D) C3b-bearing HUVECs or to (E) TNF-α–activated C3b-bearing glomerular endothelial cells in the presence of wt and mutant FH19-20 fragments. Data on mutants of specific interest to this study are shown by dark gray–shaded bars, and data on controls are shown by light gray–shaded bars. Shown are average values (± SD) of experiments performed at least 3 times. One-way ANOVA with Tukey’s multiple comparison posttests was performed. **P < .01; ***P < .001.
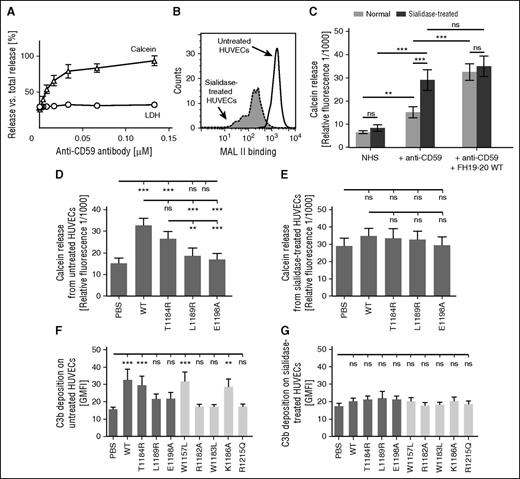
Next, a method was developed for studying complement activation. HUVECs were filled with calcein, and complement activation on cells was initiated with an anti-CD59 antibody that simultaneously activates the complement system and blocks function of the membrane-associated complement regulator CD59.28,31 Increasing anti-CD59 concentration was observed to increase calcein levels in supernatants (Figure 6A), indicating escape of the very small compound calcein (1 kDa) from the cells through complement membrane attack complex pores (C5b-9). Because the larger LDH (140 kDa) was not released (Figure 6A), the cells were not lysed. This indicated that the assay detects complement activation as a function of C5b-9 insertion in the cell membrane.
Effect of sialic acid removal on complement activation on endothelial cells incubated in serum. (A) A calcein-release assay was set up to study complement activation, and HUVECs were loaded with calcein and incubated in 15% NHS in the presence of increasing concentrations of the classical complement pathway initiator anti-CD59 antibody. Measurement of supernatant calcein fluorescence (excitation/emission wavelengths of 485/520 nm) showed a dose-dependent response for complement activation. Assessment of an LDH indicator at ex/em values 540/590 nm verified that complement activation did not lead to cell lysis with the anti-CD59 concentrations used. (B) HUVECs were incubated with sialidase or the respective buffer, and removal of sialic acid from cell surfaces was verified by showing decreased binding of MAL II lectin with flow cytometry. Shown is a representative histogram of an assay performed 4 times. (C-E) The role of sialic acid in complement regulation on HUVECs was studied by using the newly developed assay, which detected complement activation as a function of calcein released from the cells. (C) Addition of wt FH19-20 enhanced calcein release, the indicator for complement activation, on untreated but not on sialidase-treated HUVECs. (D) Complement activation on normal HUVECs in the presence of wt and mutant FH19-20 in serum sensitized with anti-CD59 antibody. (E) Complement activation on sialidase-treated HUVECs in the presence of wt and mutant FH19-20 in serum. The assays were performed 5 times. Shown are mean relative fluorescence values (± SD). (F-G) Effect of sialic acid on complement activation on HUVECs was also studied at the level of C3b deposition. (F) Untreated or (G) sialidase-treated HUVECs were incubated in 33% NHS, and FH19-20 was added to interfere with the complement-inhibitory function of FH. Fluorescently labeled C3 was included in the serum to enable later assessment of C3b deposition by flow cytometry. The assays were performed 4 times. Shown are GMFI values (± SD). One-way ANOVA with Tukey’s multiple comparison posttests was performed. **P < .01; ***P < .001.
Effect of sialic acid removal on complement activation on endothelial cells incubated in serum. (A) A calcein-release assay was set up to study complement activation, and HUVECs were loaded with calcein and incubated in 15% NHS in the presence of increasing concentrations of the classical complement pathway initiator anti-CD59 antibody. Measurement of supernatant calcein fluorescence (excitation/emission wavelengths of 485/520 nm) showed a dose-dependent response for complement activation. Assessment of an LDH indicator at ex/em values 540/590 nm verified that complement activation did not lead to cell lysis with the anti-CD59 concentrations used. (B) HUVECs were incubated with sialidase or the respective buffer, and removal of sialic acid from cell surfaces was verified by showing decreased binding of MAL II lectin with flow cytometry. Shown is a representative histogram of an assay performed 4 times. (C-E) The role of sialic acid in complement regulation on HUVECs was studied by using the newly developed assay, which detected complement activation as a function of calcein released from the cells. (C) Addition of wt FH19-20 enhanced calcein release, the indicator for complement activation, on untreated but not on sialidase-treated HUVECs. (D) Complement activation on normal HUVECs in the presence of wt and mutant FH19-20 in serum sensitized with anti-CD59 antibody. (E) Complement activation on sialidase-treated HUVECs in the presence of wt and mutant FH19-20 in serum. The assays were performed 5 times. Shown are mean relative fluorescence values (± SD). (F-G) Effect of sialic acid on complement activation on HUVECs was also studied at the level of C3b deposition. (F) Untreated or (G) sialidase-treated HUVECs were incubated in 33% NHS, and FH19-20 was added to interfere with the complement-inhibitory function of FH. Fluorescently labeled C3 was included in the serum to enable later assessment of C3b deposition by flow cytometry. The assays were performed 4 times. Shown are GMFI values (± SD). One-way ANOVA with Tukey’s multiple comparison posttests was performed. **P < .01; ***P < .001.
When a minimal amount of anti-CD59 was used to initiate complement, we were able to use the calcein-release assay to study the ability of FH19-20 to prevent FH from regulating complement on HUVECs (Figure 6C). When different FH19-20 fragments were studied, we observed a pattern similar to that of the erythrocyte and endothelial cell assays described above. The mutation T1184R caused only a small, statistically insignificant decrease in the ability of FH19-20 to compete with FH, whereas mutations L1189R and E1198A practically abolished it (Figure 6D).
Removal of sialic acid from HUVECs (Figure 6B) increased complement activation on them in serum (Figure 6C). This suggested that control by FH was reduced, but the complement-activating natural antibodies that recognize epitopes exposed during sialidase treatment could also have been involved.32,33 The problem of natural antibodies was overcome by accepting their contribution to complement activation on the sialidase-treated HUVECs, and then, on this basis, studying the additional complement-enhancing effect of FH19-20. In contrast to normal HUVECs, no further enhancement of complement activation on sialidase-treated HUVECs in the presence of wt or mutant FH19-20 was observed in the calcein-release assay (Figure 6C,E). This was not the result of the absence of further calcein release, because the level of fluorescence reached only 60% of the maximum.
The role of sialic acid in complement regulation on HUVECs was studied with another functional assay in which FH19-20 inhibition of FH-mediated regulation on HUVECs was assessed by measuring C3b deposition. Corroborating earlier results, the mutations L1189R and E1198A proved to be detrimental to the ability of FH19-20 to interfere with FH function, whereas the mutation T1184R had only a minor effect (Figure 6F). The control mutant W1157L did not affect FH19-20 function, whereas mutations W1183L, R1182A, and R1215Q completely abolished it (Figure 6F). In the absence of sialic acid, no difference between the wt and mutant FH19-20 constructs was observed (Figure 6G). Taken together, these results show that sialic acid is essential for FH-mediated regulation of complement on endothelial cells.
Platelets are protected from complement attack by sialic acid
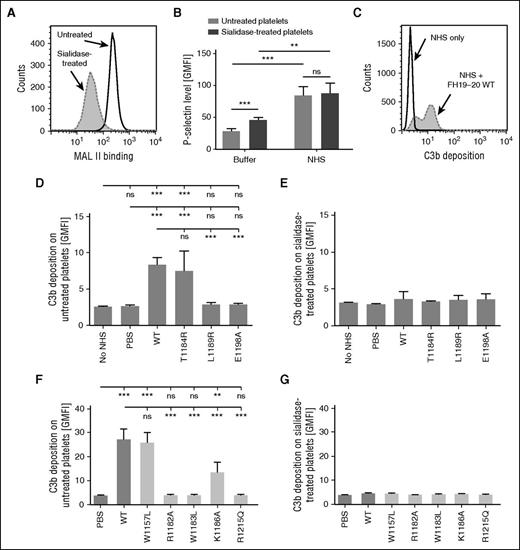
Although endothelial injury is considered central to thrombotic microangiopathies in general,23 at least in aHUS, a direct complement attack on platelets is also involved.34 To determine the role of sialic acid in complement regulation on platelets, human platelets were isolated and treated with sialidase (Figure 7A). This activated the platelets slightly, as shown by increased P-selectin expression (Figure 7B). In line with previous reports,30,35 serum exposure further activated the platelets (Figure 7B). Importantly, the difference in P-selectin expression between untreated and sialidase-treated platelets disappeared upon exposure to serum. This ruled out a major role for P-selectin in complement activation in our system.36
Effect of sialidase treatment on complement activation on platelets. (A) Washed human platelets were treated with sialidase or the respective buffer. Removal of sialic acid was confirmed by using flow cytometry to show decreased binding of fluorescently labeled MAL II. (A,C) Representative histograms of an assay performed 4 times. (B) The activation state of untreated and sialidase-treated platelets before and after serum exposure was determined by measuring P-selectin expression. Binding of anti-P-selectin antibody was detected by flow cytometry. (C-G) Platelets were incubated in 33% NHS, and FH19-20 fragments were added to disturb FH-mediated complement regulation. Fluorescently labeled C3 was included in the serum to allow measurement of complement activation as a function of deposition of fluorescent C3 fragments on platelets. (C) Addition of FH19-20 wt to serum caused an increase in C3b-deposition on untreated platelets. Shown is a representative histogram of an assay performed 5 times. (D) C3b-deposition on normal platelets incubated in serum with wt or mutant FH19-20. (E) C3b deposition on sialidase-treated platelets incubated in serum with wt or mutant FH19-20. (F-G) Assays described in (C) and (D) were also performed with control mutants FH19-20. Assays were performed 4 times. Shown are GMFI (± SD). Either (C) Student t tests or (D-G) one-way ANOVA with Tukey’ multiple comparison posttests were performed. **P < .01; ***P < .001.
Effect of sialidase treatment on complement activation on platelets. (A) Washed human platelets were treated with sialidase or the respective buffer. Removal of sialic acid was confirmed by using flow cytometry to show decreased binding of fluorescently labeled MAL II. (A,C) Representative histograms of an assay performed 4 times. (B) The activation state of untreated and sialidase-treated platelets before and after serum exposure was determined by measuring P-selectin expression. Binding of anti-P-selectin antibody was detected by flow cytometry. (C-G) Platelets were incubated in 33% NHS, and FH19-20 fragments were added to disturb FH-mediated complement regulation. Fluorescently labeled C3 was included in the serum to allow measurement of complement activation as a function of deposition of fluorescent C3 fragments on platelets. (C) Addition of FH19-20 wt to serum caused an increase in C3b-deposition on untreated platelets. Shown is a representative histogram of an assay performed 5 times. (D) C3b-deposition on normal platelets incubated in serum with wt or mutant FH19-20. (E) C3b deposition on sialidase-treated platelets incubated in serum with wt or mutant FH19-20. (F-G) Assays described in (C) and (D) were also performed with control mutants FH19-20. Assays were performed 4 times. Shown are GMFI (± SD). Either (C) Student t tests or (D-G) one-way ANOVA with Tukey’ multiple comparison posttests were performed. **P < .01; ***P < .001.
To analyze the role of sialic acid in FH-mediated regulation of complement on platelets, C3b deposition assays similar to those developed for HUVECs were performed. Addition of wt FH19-20 to platelets in serum caused an increase in C3b deposition (Figure 7C). The mutations L1189R and E1198A practically prevented this effect, while T1184R did not change it (Figure 7D). In the absence of sialic acid, differences between the wt and mutant FH19-20 fragments could no longer be observed (Figure 7E). Results of control mutants paralleled observations with HUVECs (Figure 7F-G): the W1157L and K1186A mutations had no or a minor effect on FH19-20 function, whereas R1182A, W1183L, and R1215Q proved deleterious. These results suggest that sialic acid is also involved in FH-mediated complement regulation on platelets.
Discussion
The thrombotic microangiopathy aHUS is characterized by dysregulation of the complement system.37 FH inhibits complement on self cells where it can recognize certain marker molecules. On erythrocytes, FH presumably recognizes sialic acid,15,16 whereas on endothelial cells FH binding is generally attributed to glycosaminoglycans.10 In this work, we set out to investigate why some mutations that cause increased binding of FH to heparin12,13 are associated with the disease aHUS featuring excess complement activation on cells. We showed that the pathogenicity of C-terminal FH mutations is due to impaired recognition of sialic acid on surfaces under complement attack. Sialic acid was observed to be critical for FH-mediated complement regulation not only on erythrocytes but also on endothelial cells and platelets, thus providing for the first time a unifying explanation for association of C-terminal mutations of FH with aHUS.
The main feature of aHUS is mistargeted complement attack against self cells2 with abnormal activity of this cascade causing injury for at least endothelial cells1 and platelets.34 In aHUS cases with FH mutations of the C terminus, the recognition of C3b on self cells is impaired. We investigated the reason for this by using several FH19-20 mutants that displayed various effects on the affinity of FH19-20 to heparin. The FH19-20 mutants were observed to antagonize FH function with the same pattern on all types of cells studied, that is, on erythrocytes, endothelial cells, and platelets (Figures 2, 6D-G, and 7D-G). The mutations R1182A, W1183L, L1189R, E1198A, and R1215Q proved harmful, whereas W1157L, T1184R, and K1186A had little if any effect on the ability of FH19-20 to interfere with FH-mediated complement regulation. These results are consistent with earlier data on hemolysis12 and on C3b deposition on glomerular endothelial cells.27 Importantly, our new data and recently published functional data by others12,27 were observed to be not parallel with FH19-20 binding to either heparin or C3b.12,13 Instead, the functional data paralleled that for FH19-20 binding to C3b-bearing erythrocytes and endothelial cells (Figures 4G-I and 5D-E), both of which display sialic acid on their surfaces. Careful inspection of the crystal structure of FH19-20 in complex with sialic acid and C3d17 allows us to explain these observations. Namely, the mutations W1183L and R1215Q that proved detrimental in our assays were earlier predicted to remove the two most important interactions of FH19-20 with sialic acid. Furthermore, L1189R, R1182A, and E1198A have been predicted to impair sialic acid binding to some extent,17 and our results were in line with this. Conversely, the T1184 and K1186 residues make no direct contact with sialic acid,17 and W1157 is far from the binding site,14 which explains why these mutations had either no or only a minor effect on FH19-20 function. All in all, our consistent experimental data on FH19-20 mutant behavior in various binding and functional assays with several cell types strongly supports the role of sialic acid in the physiological function of FH.
The importance of sialic acid for the regulatory function of FH was best revealed in the absence of this carbohydrate. With normal cells, adding FH19-20 to serum led to enhanced complement activation, but after endothelial cells and platelets were treated with sialidase, FH19-20 lost its ability to augment complement activation on these cells (Figures 6E-G and 7E-G). This phenomenon was previously observed on erythrocytes38 and is quite logical because, in the absence of sialic acid, FH19-20 is not expected to recognize surface-bound C3b. Thus, our functional data on complement activation against sialidase-treated cells is in favor of the importance of proper sialic acid recognition by the C terminus of FH during regulation.
Interactions of FH with endothelial cells are generally attributed to heparan sulfate. Binding of FH19-20 to kidney glomerulus sections has indeed been shown to decrease after a treatment with heparinase,10 and binding of FH19-20 to glomerular endothelial cells was recently reported to decrease on removal of heparan sulfate, although only by 15%.27 However, if heparan sulfate was significantly involved in FH function, then FH19-20 mutants L1189R and E1198A having higher affinity for heparin and for glomerular endothelial cells12,13 would be expected to display increased ability to antagonize FH function on heparan sulfate-bearing cells. Our observations were exactly the opposite: the L1189R and E1198A mutations impaired the ability of FH19-20 to inhibit FH binding to human glomerular endothelial cells bearing C3b deposition (Figure 5E). In line with this, the mutation E1198A was recently reported to abolish FH19-20 binding in a murine glomerular endothelial cell model.27 Furthermore, based on structural data, the mutations L1189R and E1198A were predicted to impair certain interactions between FH19-20 and sialic acid,17 explaining the reduced binding of the FH19-20 mutants L1189R and E1198A to C3b-bearing cells. Moreover, even though the FH6-8 construct has been reported to display higher affinity for heparin than FH19-20, FH6-8 was not able to compete with FH in binding to C3b-bearing endothelial cells (Figure 5C). All in all, our results point to sialic acid being more relevant than heparan sulfate for FH function on endothelium. However, because removal of heparan sulfate from endothelial cells has been shown to affect FH binding,27 a role of heparan sulfate in FH function cannot be completely ruled out. This negatively charged glycosaminoglycan could, for example, assist in attracting FH onto endothelium.
Impaired recognition of surface sialic acid could also explain complement dysregulation in aHUS patients with genetic rearrangements between FH and factor H-related protein 1 (FHR1).39-41 FHR1 has a C terminus identical with that of FH except for the amino acid changes to S1191L and V1197A. This double mutation was recently predicted to prevent FH19-20 from binding to sialic acid,17 suggesting one explanation for the pathogenesis of FH-FHR1 hybrids. The FH/FHR1 hybrid40,41 contains the N terminus of FH capable of complement regulation, but because the C terminus originating from FHR1 is unable to guide the molecule onto cell surfaces under complement attack, cell protection does not occur. Conversely, the reverse hybrid FHR1/FH42 is efficiently directed onto cells by the C terminus originating from FH, but the N-terminal FHR domains lack regulatory function.43 Thus, the FHR1/FH hybrid acts as an antagonist of FH and causes increased complement activation on erythrocytes and endothelial cells.40 Taken together, disturbances in sialic acid recognition provide one explanation for the pathogenicity of various FH-FHR1 hybrids.
In conclusion, the data presented in this article show that FH-mediated complement regulation necessitates the presence of sialic acid on cells. We propose that in aHUS, the underlying common defect of various FH C terminus mutations is the inability to simultaneously bind sialic acid and C3b on erythrocytes, endothelial cells, and platelets. Reduced control over complement on these cells explains not only hemolysis but also endothelial cell damage and thrombus formation leading to thrombotic microangiopathy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mikko Helenius and Tero-Pekka Alastalo for the human umbilical vein endothelial cells, Marjo Rissanen for general technical assistance, Arja Pasternack for the laminar hood facility, and Derek Ho for language editing.
This work was supported by the research grants from the Sigrid Jusélius Foundation and grants from the Academy of Finland (128646 and 259793).
Authorship
Contribution: S.H. and T.S.J. conceptualized the study; S.H. designed and performed the experiments, analyzed the results, and wrote the manuscript; and S.M. and T.S.J. critically commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satu Hyvärinen, Immunobiology Research Program, University of Helsinki, Haartmaninkatu 3, FIN-00014 Helsinki, Finland; e-mail: satu.hyvarinen@helsinki.fi.

![Figure 1. FH19-20 residues involved in binding to C3b and sialic acid. Shown are mesh-and-sphere representations of the FH19-20 structure (Protein Data Bank [PDB] ID code 2G7I)14 from two different angles. (A) Binding sites for C3b/C3d and sialic acid. The main interface residues forming the C3b binding site (N1117, Q1139, Y1142, P1166, K1188, and Y1190)21,22 are shown in green. The residues likely to have an impact on sialic acid binding (I1169, R1182, T1184, L1189, S1191, G1194, V1197, E1198, F1199, and R1215)17 are shown in dark yellow. Residues of specific relevance to this study (T1184, L1189 and E1198) have been highlighted with orange. (B) Location of the residues that were mutated in the control constructs. Whereas the aHUS-associated mutations T1184R, L1189R, and E1198A lead to increased FH19-20 affinity for heparin, other mutations have been reported to either decrease (R1182A, K1186A, and R1215Q) or have no effect (W1157L, W1183L) on the interaction.12,13 Residues R1182, W1183, K1186, and R1215 are depicted in blue. Residue W1157 is invisible because it is buried within the domain 19.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/22/10.1182_blood-2015-11-680009/4/m_2701f1.jpeg?Expires=1768497425&Signature=39EgumCKSpBTkvjFJxQGCa7LHRznR2l~Jrs4ToNHZQ4T81diQOT7E7UraoDdo2pyMVB5Rqn-~ufmEK4hkJq9bdicsptpMAXzG9o0RZi-8KxigegZKEtdQpOFk6rmTaLeymRrQ0mvLAEsJx8hbgEhDH9zZZtvmO-jeJmm03u3qz6hHbde-hWMMlRrRqG2E6HJl-ez3VYXV0MmROU30Y2-0JRXT3hAIew-ExBkg5gCdR8NboNuCvAMxQ6cetPfd3Xqf7u8jM6-hqEYIQL-4NC979kGJbYbjiZWJOoUrZ4LoKObismJKAIsrRQr8BArDZLCFlENpCLLHnM6-5xXrJ3Dcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal